Research - (2021) Volume 9, Issue 6
A Comparative Study of Quantitative Buffy Coat (QBC) and Rapid Diagnostic Test (RDT) Against the Conventional Peripheral Blood Smear Microscopy in the Early Detection and Diagnosis of Malaria in Children
Shachi Bhanuda, M Shafath Ahmed and S Sundari*
*Correspondence: S Sundari, Department of Orthopaedics, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, India, India, Email:
Abstract
The Present study includes a comparative study evaluating the diagnostic value of Quantitative Buffy Coat and Rapid Diagnostic Test (using pLDH) against the conventional peripheral blood smear microscopy, in the diagnosis of malaria in children between 6 months to 14 years of age. The present study focuses on the clinical profile of malaria cases detected by the peripheral smear. RDT (pLDH) is highly sensitive in picking up falciparum cases, which is equivalent to QBC. So, it can be used as a diagnostic tool for falciparum malaria where facilities of expert microscopy/ QBC are not available. The sensitivity of RDT (pLDH) in diagnosing vivax malaria is lower than that of peripheral smear microscopy and QBC.
Keywords
Malaria, Anemia, RDT, QBC, Falciparum, Microscopy
Introduction
Malaria is an endemic disease of tropics and subtropics and one of the major public health problems worldwide. Despite making considerable advances in the treatment and prevention of malaria, it still continues to threaten the lives of millions of people. As indicated by the World Malaria Report 2018, approximately 219 million cases of malaria occurred globally in 2017, compared to 239 million cases in 2010, of which 435,000 people died of malaria-related complications, a decrease from 607,000 deaths in 2010. Approximately 90% of these cases and deaths occurred in the regions of Sub-Saharan Africa. Children aged less than 5 years sustain most suffering with maximum number of mortalities. In 2017, an estimated 266,000 children under 5-year age group died of malaria, accounting for 61% of all malarial deaths globally. It is estimated that every two minutes, a child loses his life because of malaria.
This year’s report also includes a segment on malariaassociated anemia, which was once a key measure of progress in control of malaria, and its prevalence was useful to assess the effectiveness of interventions. There has been a decline in the awareness of burden of malariarelated anemia in the recent years. Of children who showed positive results for malaria, the prevalence of any anaemia was 79%, mild anemia 21%, moderate anemia 50% and severe anemia 8%.
Etiology
There are four species of protozoan Plasmodium genus that are known to cause malaria: P. vivax, P. malariae, P. ovale, P. falciparum. Infection by P. vivax is the commonest whereas by P.falciparum is the most serious and responsible for deaths among children. The sporozoite is transmitted to the host by Anopheline mosquito. Transmission may also occur transplacentally and rarely through blood transfusion. In India, the most common species causing malaria are P. falciparum and P. vivax. P. vivax prevails more commonly in the plain areas, whereas P. falciparum is mainly found in forests and hilly areas.
The magnitude of this problem is further complicated by P. falciparum resistance found against standard antimalarial drugs, increasing associated mortality and morbidity [1-5]. Therefore, our goal is to keep indiscriminate use of antimalarial drugs under control and ensuring uniform prescribing practices to curb the spread and aggravation of drug resistance. One way of achieving this is by improving the diagnostic practices of malaria.
Although microscopy is considered as the gold standard method for diagnosing malaria, it is time consuming, labour intensive and needs technical expertise for the interpretation of its results. It also has the drawback of variable sensitivity and specificity when compared to the recent advancements, especially at low levels of parasitemia. Developing countries play a major role in contributing to the burden of the disease, hence costeffectiveness of the test and urgency of obtaining the results in suspected malaria cases are of utmost importance, which is difficult to achieve with some of the current sensitive methods of diagnosis. Thus, availability of a simple, rapid and accurate test, particularly in remote areas which lack adequate health facilities, could be of great help. In this study, we are evaluating the diagnostic value of QBC and RDT (pLDH) in comparison with the gold standard peripheral smear microscopy for diagnosis of malaria in children.
Life cycle of malaria
Plasmodium multiplies in a two-step process in humans, with the first phase occurring in the hepatic cells (exoerythrocytic phase) and the second phase in the RBCs (erythrocytic phase).
During a blood meal, plasmodium-infected female Anopheles mosquito inoculates the sporozoites into bloodstream of the human host (1), which enter hepatocytes of liver within minutes (2) where they develop and multiply asexually into a schizont (3). After 1-2 weeks, this schizont ruptures and releases the merozoites into circulation (4). This is the exoerythrocytic schizogony (A).
The erythrocytic phase (B) begins when these merozoites from the liver infect RBCs (5). The parasite transforms into ring form, which enlarges to become trophozoite. They multiply asexually to produce erythrocytic merozoites which are then released into bloodstream (6). Few of these parasites differentiate into gametocytes, the sexual erythrocytic stage (7). Erythrocytic schizogony is responsible for clinical manifestations of malaria. During blood meal, the microgametocyte (male) and macrogametocyte (female) are ingested by female Anopheles mosquito (8) and undergo further multiplication and transformation called the Sporogonic cycle (C). In the stomach cavity of the mosquito, the male and female gametocyte fuse to form a zygote (9) which matures into motile ookinete (10). These ookinetes invade mid-gut wall and develop into oocysts (11), which rupture and release sporozoites (13). These sporozoites reach salivary glands of mosquito and get injected into a new human host when mosquito takes its blood meal.
Diagnosis of malaria
Clinical diagnosis
The classic presentation of malaria consists of the paroxysms of fever alternating with the periods of fatigue, but otherwise relative wellness. The febrile paroxysms are characterized by high fever, rigors, sweats, headache, myalgia, abdominal pain, back pain, nausea, vomiting, diarrhoea, pallor, jaundice, splenomegaly, hepatomegaly, thrombocytopenia, a normal or low leukocyte count, elevated ESR or any combination of these manifestations. Paroxysms coincide with rupture of schizonts which occurs every 48 hrs with P. vivax and P. ovale, resulting in the appearance of fever spikes every other day. Rupture of schizonts occurs every 72 hrs with P. malariae and results in fever spikes every third or fourth day. Periodicity is less apparent with P. falciparum and mixed infections. Severe, high risk malaria is characterized by impaired consciousness, seizures, respiratory distress or airway obstruction, hypoxia, tachycardia, hypotension, dehydration, metabolic acidosis, hypoglycemia, and hyperkalemia.
Because of vague signs and symptomology of malaria which overlaps with other common tropical infections, a clinical diagnosis of malaria is challenging to make. This overlapping impedes diagnostic specificity, which can ultimately result in indiscriminate use of antimalarial and compromise the care of patients with non-malarial fevers (Figure 1 and Figure 2).
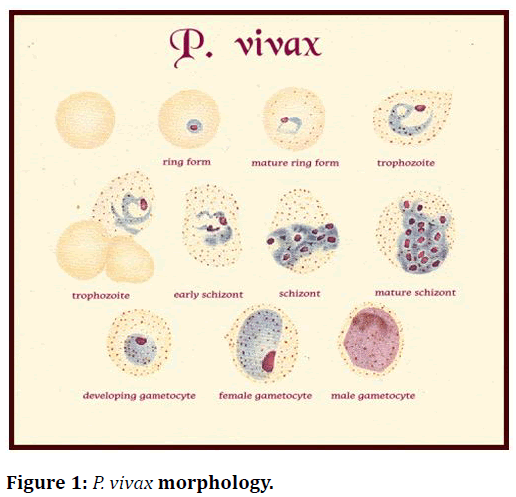
Figure 1. P. vivax morphology.
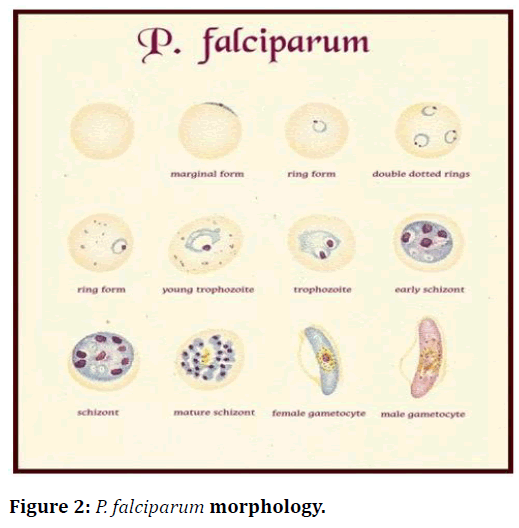
Figure 2. P. falciparum morphology.
Diagnostic points
• RBCs containing parasites are usually enlarged.
• Frequently, Schuffner’s dots are present in RBCs.
• Mature ring forms tend to be coarse and large.
• The developing forms are frequently present.
Materials and Methods
Study design: Descriptive cross-sectional study.
Study place: Department of Paediatrics at Sree Balaji Medical College and Hospital, Chromepet, Chennai
Study period: April 2018-March 2019.
Inclusion criteria
• Children between 6 months to 14 years of age.
• Children with history of fever and other clinical signs and symptoms suggestive of malaria like fever with chills and rigors, pallor, anemia, hepatosplenomegaly etc.
• Children with uncomplicated malaria.
Exclusion criteria
• Children below the age of 6 months and above the age of 14 years.
• Children with severe clinical manifestations who required immediate referral.
• Fever with obvious focus of infection like abscess, urinary tract infection (which also causes fever with chills).
• Children who had received treatment for malaria within past 4 weeks.
• Sample size: 100 children satisfying the case definition and inclusion criteria were selected and subjected for peripheral smear, QBC & RDT (pLDH).
Manoeuvre
Children were enrolled on the basis of inclusion and exclusion criteria after obtaining parental consent. Using patient data entry form, information was obtained regarding history and complaints. They were clinically examined and under aseptic precautions, about 3 ml of blood sample was collected by venipuncture. The following investigations were carried out using the sample: QBC and RDT (pLDH) apart from routine investigations. For peripheral smear (thick and thin) examination, capillary blood was obtained by finger prick method.
Peripheral smear study
The smears were obtained as per standard technique and stained with Leishman’s stain. A minimum of 200 oil immersion fields (x 100 objective) were examined in thick film. Following the detection of malarial parasites in a thick film, the thin film was examined to determine the species. If the malarial parasites were absent in the thick smear, the entire thin film was examined.
QBC
For QBC technique, approximately 60 μL of blood was taken into a capillary tube coated with acridine orange from black lined end and fitted with a cap. Then, a plastic float was inserted inside the capillary tube and centrifugation was done. The tube was then mounted on a small plastic holder and examined by rotating the tube under ordinary light microscope with customized fluorescence.
RDT (pLDH)
Plasmodium lactate dehydrogenase immunochromatographic assay was done using the commercial kit DiaMed OptiMal-IT (flow,inc.,Portland,oreg).The device was placed horizontally on a flat surface and patients name and date was written on the label. One drop of buffer to first (conjugate) well, and four drops to second (wash) well was added and waited for 1 minute. Blood was taken up to the black mark on the pipette. Entire volume of blood (10μml) was added to the first well. The mixture was gently stirred with the upper end of the pipette and allowed to stand for 1 minute, during which the lysis buffer disrupts the RBCs to release pLDH. The dipstick holder was pulled out, the legs of the dipstick holder was inserted into the holes besides the conjugate well, so that the dipstick end reaches the bottom of the conjugate well. For the next 8 min the blood/conjugate mixture is allowed to migrate to the top of the pLDH strip. The dipstick is transferred to the second well with the washing buffer which clears the hemoglobin from the strip. Once the reaction field is completely cleared of blood, and the control band is clearly visible, the dipstick was removed from the wash well and fixed back into the clear plastic frame & observed for the presence of any band and the corresponding letter C,P and Pf. Interpretation of the test result was done as below:
• When one control band and two test bands (genus specific, species specific) appeared, the test was considered to be positive for P. falciparum.
• When one control band and one test band appeared the test was considered positive for P. vivax.
• When only control band appeared without test band the test was considered to be invalid.
• Mixed infection with P. falciparum and other plasmodium species is identified by the presence of both genus specific, species specific bands. The genus specific band is much darker than the species specific band. Peripheral smear, RDT, QBC were performed by 3 different persons for the sake of blinding. Investigation results were tabulated and analyzed.
Statistical analysis
Data was entered in microsoft office excel sheet and statistical operations were performed using SPSS statistical software (Version 24). Chi-square test was used to observe statistical difference among the observed value and experimental value of variables. All the difference would be considered statistically significant at p-value <0.05. Sensitivity, specificity, positive predictive value, negative predictive value were calculated for QBC and RDT (pLDH) by comparing the results with the gold standard peripheral smear study.
Results
Out of 100 cases, 42 had come positive for malarial parasite by peripheral blood smear examination. They are confirmed cases of malaria since smear examination is considered as gold standard method for diagnosing malaria. Among the 42 cases, 38 were positive for vivax and only 4 were positive for falciparum malaria. Of 100 children, 53 children were detected by RDT out of which 48 were vivax and 5 were falciparum cases, and 55 children were detected by QBC in which 50 were vivax and 5 were falciparum malaria cases (Table 1 and Figure 3).
| 1 | Peripheral smear (PS) positive cases | 42 |
| P. vivax | 38 | |
| P. falciparum | 4 | |
| 2 | Rapid Diagnostic Test (RDT) positive cases | 53 |
| P. vivax | 48 | |
| P. falciparum | 5 | |
| 3 | Quantitative Buffy Coat (QBC) positive cases | 55 |
| P. vivax | 50 | |
| P. falciparum | 5 |
Table 1: Positive cases of peripheral smear, RDT and QBC.
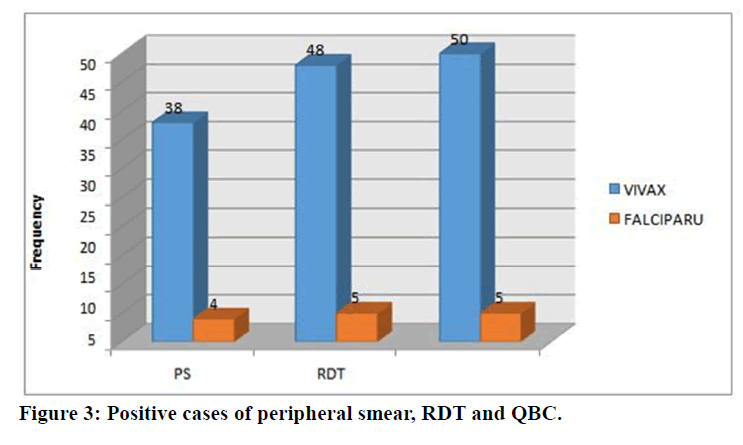
Figure 3. Positive cases of peripheral smear, RDT and QBC.
Out of the 100 studied cases, 42% were smear positive malaria, 55% were detected by QBC method and 53% were diagnosed by RDT (Table 2). Clinical features and investigations of the 42 peripheral Smear positive results are shown in table 3.
| Tests | Results | P. vivax N(%) | P. falciparum N(%) | Total (N=100) |
|---|---|---|---|---|
| Peripheral smear | Positive | 38(90.4%) | 4(9.5%) | 42(42%) |
| QBC | Positive | 50(90.9%) | 5(9.1%) | 55(55%) |
| Negative | - | - | ||
| RDT | Positive | 48(90.6%) | 5(9.4%) | 53(53%) |
| Negative | - | - |
Table 2: Comparison of QBC and RDT with peripheral smear positive results- species wise.
| S. No. | Clinical features | Frequency | Percentage |
|---|---|---|---|
| 1 | Fever | 42 | 100% |
| 2 | Chills | 9 | 21.40% |
| 3 | Headache | 7 | 16.70% |
| 4 | Myalgia | 6 | 14.30% |
| 5 | GIT symptoms | 18 | 42.90% |
| 6 | Pallor | 22 | 52.40% |
| 7 | Icterus | 2 | 4.80% |
| 8 | Edema | 0 | 0 |
| 9 | Hepatomegaly | 5 | 11.90% |
| 10 | Splenomegaly | 10 | 23.80% |
| 11 | HSM | 26 | 61.90% |
| 12 | Anemia | 28 | 66.70% |
| 13 | Thrombocytopenia | 25 | 59.50% |
Table 3: Clinical features and investigations of the 42 peripheral smear positive results.
Of the selected cases, 37% of the children belonged to the age group of 6 mo-5 years, 48% belonged to 5-10 years age group and 15% belonged to >10 years age group. The youngest patient was 6 months old and the oldest was 14 years of age. The mean age was 6.84 and the standard deviation is 3.174 (Table 4 and Figure 4). Gender distribution is mentioned in Table 5.
| Age group | Number of patients | Percentage (%) |
|---|---|---|
| 6 months-5 years | 37 | 37% |
| 5-10 years | 48 | 48% |
| >10 years | 15 | 15% |
| Total | 100 | 100% |
Table 4: Age distribution of selected cases.
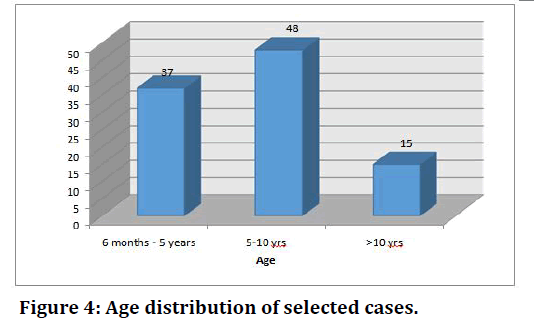
Figure 4. Age distribution of selected cases.
| Sex | Number of patients | Percentage (%) |
|---|---|---|
| Male | 57 | 57% |
| Female | 43 | 43% |
| Total | 100 | 100% |
Table 5: Sex distribution of selected cases.
Discussion
In our study, the clinical suspicion of malaria was made in 100 children. Among them, only 42 were diagnosed to have malaria on the basis of smear positivity.
As the symptoms and the signs of malaria are vague, the clinical diagnosis of malaria is very difficult and sometimes an over-enthusiastic diagnosis made solely on clinical grounds, lead on to unnecessary use of antimalarial drugs which has led to emerging drug resistance in malaria.
So, we have to depend on the laboratory investigations for accurate diagnosis.
Age group
Majority of cases of malaria in our study were between 5 to 10 years of age group, which is in contrast with the previous studies where the main affected group was between 6 months to 5 years.
Gender
Male to female ratio is 1.32:1 which was similar to the previous studies [6-12].
Clinical features
Fever was present in all (100%) cases of confirmed malaria on the day of admission. The characteristic fever with chills and rigor was observed in 21.4% of patients only, which was in contrast with Trape et al. [13] study where it was present in 51.7% cases, making it the most common symptom next to fever. In our study, the second most common complaints were GIT symptoms (42.9%) in the form of nausea, vomiting, anorexia, abdominal pain and loose stools. 16.7% of cases had headache and 14.3% had muscle ache.
On examination, pallor was observed clinically in 22 (52.4%) cases. Icterus was noted in 2 (4.8%) cases. This is caused due to hemolysis as evidenced by low haemoglobin levels in them. 5 (11.9%) patients had hepatomegaly only, 10 (23.8%) had splenomegaly only, 26 (61.9%) had both hepatosplenomegaly.
The majority of children with malaria in our study, had pallor, GIT symptoms and hepatosplenomegaly in addition to fever which was similar to the earlier observation by Fleischer et al. [14-16].
Investigations
Among the malaria cases, 66.7% of the patients had anemia with 52.5% having hemoglobin levels of 7-10 gm % and 14.2% had <7 gm%. In our study, the incidence of anemia in malaria detected by peripheral smear is more when compared to the previous studies whose predominant study population with malaria was between 9-12 years of age. The increased incidence of anemia in our series may be explained by the superadded nutritional anemia observed in younger children. There was no requirement of blood transfusion in any case.
Among the malaria cases detected by smear, 59.5% had platelet count of <1.5 lakh which is similar to the study performed by Jadhav et al.18 and in contrast with previous study showed thrombocytopenia in only 25% of cases.
Evaluation of tests
Time requirement.
Peripheral smear: 60 to 90 minutes.
QBC: Parasites can be detected within few minutes in a positive sample. About 10 minutes are required for a negative sample. (total 15 minutes, including centrifugation).
RDT(pLDH) : 10 minutes.
Of the 100 cases, 42 (42%) cases were positive for malaria and 58 (58%) cases were negative by peripheral smear microscopy. Out of 42 malaria cases, 38 (90.4%) were positive for P. vivax and 4 (9.5%) were positive for P. falciparum. QBC test was positive in 55 cases and RDT (pLDH) was positive in 53 cases. The sensitivity, specificity, positive predictive value, negative predictive value of RDT for P. vivax and P. falciparum were compared with peripheral smear.
QBC analysis
The QBC detected 55 cases of malaria of which, 50 (90.9%) were positive for P. vivax and 5 (9.1%) were positive for P. falciparum. The cases which were positive by smear, were also detected by the QBC method. In addition, 13 cases which were not detected by smear, were diagnosed as malaria by QBC technique which includes one P. falciparum case. All patients who were malaria parasite negative by QBC method were also smear negative.
The QBC showed sensitivity of 100%, specificity of 77.59%, positive predictive value of 76.36% and negative predictive value of 100% for both vivax and falciparum malaria infections in comparison with smear. This is comparable to the observations made by Trape et al. [13] who reported results of higher sensitivity of 97.5% and 96.7% respectively for QBC.
The QBC method showed sensitivity, specificity, positive predictive value and negative predictive value of 100%, 80.65%, 76%, 100% respectively for P. vivax and 100%, 98.96%, 80%, 100% respectively for P. falciparum. The results of P. falciparum are consistent with Trape et al. [13] study where they reported higher sensitivity and specificity of 100% and 95.8% respectively for detection of falciparum by QBC . Similar to the earlier observations by WHO [14], Cooke et al. [17] our study identified 13 cases of probable malaria (including one P. falciparum case) which were negative by peripheral smear. These patients were treated with antimalarial drugs and showed good clinical improvement. The reason for smear negativity may be because of low parasitemia as observed by WHO [14] and Trape et al. [13]
Rapid diagnostic test analysis (OptiMAL-IT using pLDH)
The RDT (pLDH) test showed 53 cases were positive for malaria. Amongst the 53 cases, 48 (90.6%) were positive for P. vivax and 5 (9.4%) were positive for P. falciparum. A case which was negative by RDT (pLDH) was found to be positive by smear as well as QBC. This could be attributed to low antigen levels as observed by Iqbal et al.18 or insufficient enzyme production which occurs during early malarial infection. This test identified 12 additional cases of malaria which were negative by peripheral smear. All these 12 cases were also positive by QBC and showed good response to antimalarial drugs.
One case of P. falciparum was missed by peripheral smear method, found to be positive by both QBC and RDT methods. No mixed infection was identified by any of these methods. No mortality has been observed in our study. RDT showed sensitivity of 97.62%, specificity of 79.31%, positive predictive value of 77.36% and negative predictive value of 97.87% for both vivax and falciparum malaria infections in comparison with smear, which coincided with the study of Arijit Majumdar et al.13, where they reported sensitivity of 91.6% and specificity of 75% for RDT [18-25].
The RDT method showed sensitivity, specificity, positive predictive value and negative predictive value of 97.37%, 82.26%, 77.08%, 98.08% for P. vivax and 100%, 98.96%, 80%, 100% for P. falciparum. The sensitivity rate is comparable to the previous studies.
Conclusion
QBC and RDT are equivalent in comparison with peripheral smear examination for detection of suspected cases of malaria. QBC and RDT also identified additional cases of malaria which were not picked up by the smear and showed high sensitivity for P. falciparum cases. Hence, in future, the peripheral smear examination can be replaced by Quantitative Buffy Coat technique for the diagnosis of malaria.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledegements
The encouragement and support from Bharath University, Chennai is gratefully acknowledged. For provided the laboratory facilities to carry out the research work.
References
- https://www.who.int/publications/i
- Ritabrata Kundu. Malaria. IAP textbook of pediatrics. 2013; 5:221.
- https://nvbdcp.gov.in/index4.php?lang=1&level=0&linkid=420&lid=%203699
- https://tnhealth.tn.gov.in/tngovin/dph/dphpm.php
- Barnish G, Bates I, Iboro J. Newer drug combinations for malaria. BMJ 2004; 328:1511–1512.
- https://www.cdc.gov/malaria/about/biology/index.html
- https://www.us.elsevierhealth.com/nelson-textbook-of-pediatrics-2-volume-set-9780323529501.html
- Castelli F, Carosi G. Diagnosis of malaria infection. Handbook of malaria infection in the tropics. 1997; 15:114.
- Warhurst DC, William J. Laboratory diagnosis of malaria ACP broadsheet No:148. J Clin Pathol 1996; 49:533-8.
- Long GW, Jones TR, Rickman LS, et al. Acridine orange diagnosis of plasmodium falciparum; evaluation after Experimental infection. Am Society Tropical Med Hygiene 1994; 51:613-16.
- Payne D. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull World Health Organisation 1988; 66:621-628.
- Anonymous. The laboratory diagnosis of malaria. The malaria working party of the general haematology task force of the british committee for standards in haematology. Clin Lab Haematol 1997; 19:165-170.
- Trape JF. Rapid evaluation of malaria parasite density and standardization of thick smear examination for epidemiological investigations. Trans R Soc Trop Med Hyg 1985; 79:181–184.
- World Health Organization. Basic Malaria Microscopy. Geneva: WHO 1991.
- Fleischer B. 100 years ago: Giemsa’s solution for staining of plasmodia. Trop Med Int Health 2004; 9:755–756.
- Srinavasan S, Moody A H , Chiodini P L. Comparison of blood- film microscopy, the optimal® dipstick, rhodamine 123 and PCR for monitoring anti-malarial treatment. Ann Trop Med Parasitol 2000; 94:227–232
- Cooke, Morris-Jones S, Horton J, et al. Evaluation of benzothiocarboxypurine for malaria diagnosis in an endemic area. Trans R Soc Trop Med Hyg 1993; 87:549.
- https://www.worldcat.org/oclc/61362865
- Krishna B V, Deshpande AR. Comparison between conventional and QBC methods for diagnosis of malaria. Indian J Pathol Microbiol. 2003; 46:517-20.
- Laferl H, Kandel K, Pichler H. False positive dipstick test for malaria. N Engl J Med 1997; 337:1635–1636.
- https://journals.asm.org/doi/abs/10.1128/aac.46.6.1658-1664.2002
- Mishra B, Samantaray JC, Kumar A, et al. Study of false positivity of two rapid antigen detection tests for diagnosis of plasmodium falciparum malaria. J Clin Microbiol 1999; 37:1233.
- Laferl H, Kandel K, Pichler H. False positive dipstick test for malaria. N Engl J Med 1997; 337:1635–1636.
- Grobusch MP, Apermann U, Schwenke S, et al. False-positive rapid tests for malaria in patients with rheumatoid factor. Lancet 1999; 353:297-99.
- Mishra B, Samantaray JC, Kumar A, et al. Study of false positivity of two rapid antigen detection tests for diagnosis of plasmodium falciparum malaria. J Clin Microbiol 1999; 37:1233.
Author Info
Shachi Bhanuda, M Shafath Ahmed and S Sundari*
Department of Orthopaedics, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, India, IndiaCitation: Shachi Bhanuda, M Shafath Ahmed, S Sundari, A Comparative Study of Quantitative Buffy Coat (QBC) and Rapid Diagnostic Test (RDT) Against the Conventional Peripheral Blood Smear Microscopy in the Early Detection and Diagnosis of Malaria in Children, J Res Med Dent Sci, 2021, 9(6): 294-300.
Received: 30-Mar-2021 Accepted: 23-Jun-2021
