Research - (2022) Volume 10, Issue 6
A Comparative Study on the Biochemical Variation on Women with Polycystic Ovaries
Pavithra Ramakrishna, Kalpana Thalava and G Jayalakshmi*
*Correspondence: G Jayalakshmi, Department of Obstetrics and Gynaecology, Sri Lakshmi Narayana Institute of Medical Sciences Affiliated to Bharath Institute of Higher Education and Research, India, Email:
Abstract
The most prevalent women associated with chronic is polycystic-ovarian syndrome (PCOS), which affects 5-10% of females of childbearing age. In our study the most modifiable factor was obesity and over us right that constitutes 70 % of the cases, hence the importance of weigh t reduction in the management of pcos is very important. Serum prolactin and thyroid function tests were done. Serum LH and LH/F SH ratio do not prove to be a significant additional diagnostic too1. Transvaginal ultrasound acts as an important tool in diagnosing PCOS. In our case the three main symptoms were menstrual irregularities, hyperandrogenism and infertility.
Keywords
Anovulation, Hyperandrogenism, Infertility, Insulin resistance, Hirsutism
Introduction
A most prevalent woman endocrinopathy is polycystic ovarian syndrome (PCOS), which affects 5-10% of women of childbearing age. It is a heterogeneous endocrine and metabolic disorder, associated with menstrual disorders, infertility, obesity, hirsutism and insulin resistance. The spectrum of disorder varies from a mild presentation to a severe disturbance of reproductive, endocrine & metabolic function. The knowledge has progressed from old notions of infertility as primarily a gynecological problem to more recent concepts that include a metabolic element marked by insulin resistance, a probable hereditary etiology, and possibly distressing long-term health consequences. It is obvious that the disease cannot be simply described by a single unifying concept, and that the symptoms of PCOS are most likely caused by a complex interaction of genetic, epigenetic, and environmental variables [1-4].
New advances in biotechnology (microarrays and proteomics) and bioinformatics should help us develop comprehend this intricacy. While the clinical presentation usually indicates the identification, lab testing is required to rule out other illnesses that may resemble PCOS. PCOS is diagnosed mostly by clinical history and physical examination results. Many normal, healthy women in the regular populace will have the characteristic ultrasound look of polycystic ovaries but no related symptoms, and nothing is known about their natural history or health risks. It's important to distinguish between women who have polycystic ovaries but no symptoms and those who have enough symptoms and indicators to be diagnosed with PCOS [5,6].
Materials and Methods
PCOS should contain a minimum 12 follicles ranging 2-9 mm in diameter, as well as enhanced ovarian capacity (>10 cm3). Follicle number more than 2 with follicle size 2-9mm Ovarian volume (>10cm 3 ) was calculated with formula of prolate ellipsoid =0.5x length x breadth x thickness. Average ovarian volume was taken as reference. Blood samples were tested in the early follicular phase on day 3 for Serum LH, FSH & Total Testosterone. Serum LH, FSH & Total Testosterone was done by ECLIA (Electrochemiilluminiscence immunoassay).
Results
In the present study 20% of cases were lean/normal weight with BMI<25. Overweight was seen 45% cases and 35% were found to be obese (Figure 1).
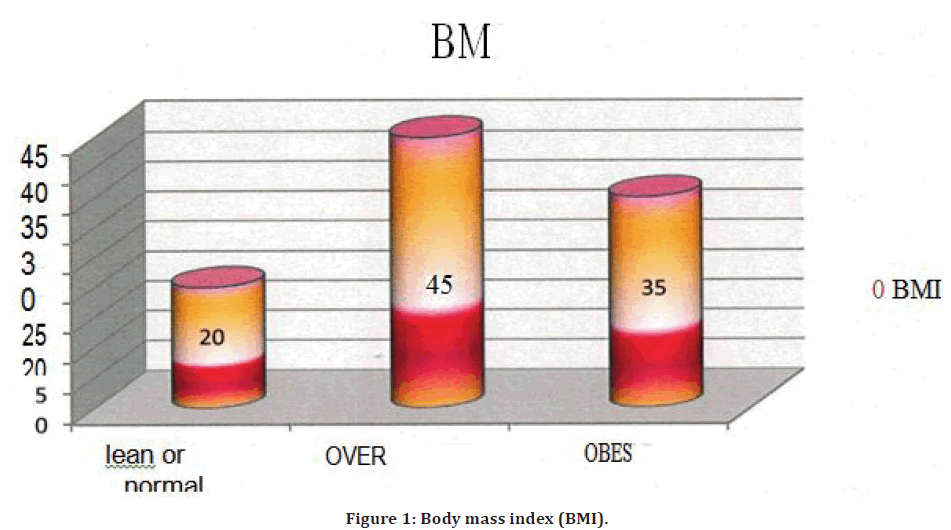
Figure 1. Body mass index (BMI).
For all PCOS cases we checked the thyroid profile. FT3, FT4, TSH values were checked, in that 17 cases were found to be having hypothyroidism with menstrua1 irregularities (Figure 2). 7 cases that correspond to 41.1% cases had oligomenorrhea with menorrhagia. Nad 10 cases that correspond to 58.9 cases had oligomenorrhea. In this study FSH/ LH ratio was < 3 in 60% of the cases and was > 3 in only 40% cases. The mean FSH/LH ratio in the present study was 3.3.
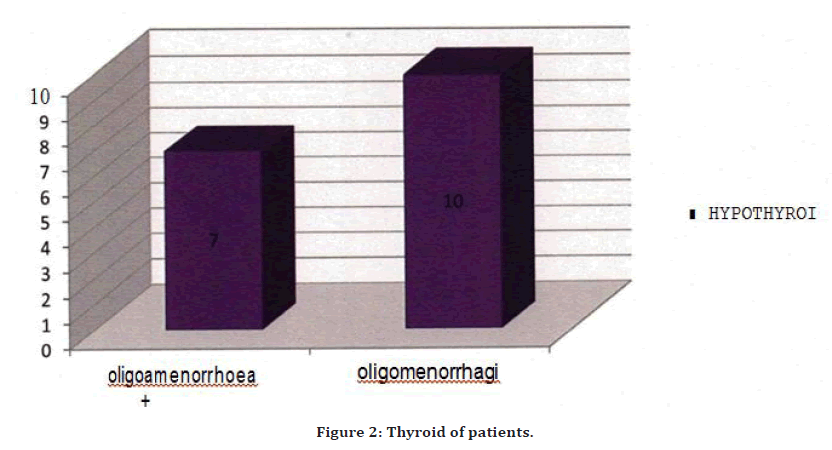
Figure 2. Thyroid of patients.
In the present study 61% had Serum LH value > 10mIU/ ml. 395 had Serum Li. than 10mIU/ml. The mean value of Serum LH was 12.9mIU/ml with a standard deviation of 6.0. In the present study, with statistical analysis, there was no significant correlation between Serum LH with oligo/amenorrhoea, hirsutism and acne (Figure 3).
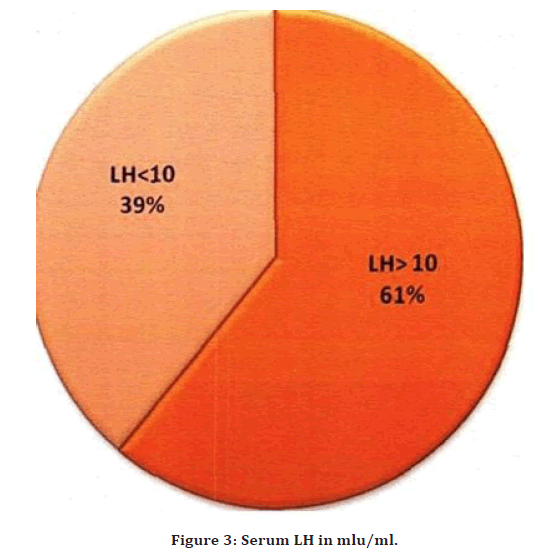
Figure 3. Serum LH in mlu/ml.
In the present study, serum LH (> l 0mIU/ml) was elevated in 55% patients. In the Revised 2003 consensus it was concluded that both the absolute levels of circulating LH as well as its relation to FSH levels are significantly elevated in women with PCOS. Based on the aforementioned data, the panel felt that measurement of serum LH 1eve1s should not be considered necessary for the clinical diagnosis of PCOS. LH levels could be usefu1 as a secondary parameter (especia1ly in lean women with amenorrhea or in research).
Discussion
They conclude that all women with PCOS, regard less of weight or BMI, should work to prevent cardiovascu1ar complications. In the present study we observe d that 9% of cases had elevated serum prolactin value >20ng/ ml. Carlos Andres Valverde, MD, her study found, PCOS occurs in upto 10% of reproductive age women. It is the most common etiology underlying ovulatory problems. Several diagnostic criteria are used, but the most widely accepted definition of the syndrome is chronic anowitation or oligoovulation and clinical or lab oratory hyperandrogenemia in the absence of other sources of androgen excess. Hyperprolactinemia is a less frequent cause of menstrual abnormalities, with a reported prevalence of 0.4 percent to 17%. Women with PCOS have been shown to have a higher incidence. Because hyperprolactinemia can cause increased androgen 1evels, it must be ruled out before the confirmation of PCOS can be confirmed.
The only anterior pituitary hormone that is constantly suppressed is prolactin. Dopamine is created in the hypothalamus and travels through the pituitary porta1 circulation to the anterior pituitary, where it reduces pro1actin secretion. When this suppression is disrupted, hyperprolactinemia develops. Because prolactin production follows a circadian pattern and varies during the menstrual cycle, it should be tested at least twice. Pregnancy, nipple stimulation, breastfeeding, stress, eating, thyroid problems, and certain medicines can all cause hyperpro lactinemia, therefore they must be taken into account while assessing hyperpro lactinemia. The most prevalent cause of chronic hyperprolactinemia is pituitary adenomas, which must be checked out. Prolactin inhibits gonadotropin-releasing hormone agonist (GnRH) production, which can lead to a variety of ovulatory issues. Pro1actin, at sufficiently high levels, can cause amenorrhea in severe situations.
Prolactin synthesis is stimulated by estrogen. Women with PCO S frequently have persistently increased estradiol levels, which can lead to modest pro1actin elevation. The research comprised 82 women with PCOS, ranging in age from 27.1 to 7.6 years. Their PRL values were compared to those of women without PCOS who had insulin resistance (contro1s; o42; age: 29.2 ± 8.2 years). Thirteen (16%) of the 82 PCOS women had high PRL 1eve1s (103.9 ± 136.0 pg/1) 3' As a result of this finding, we believe that PCOS patients with elevated PRL levels should be checked for potential causes of hyperprolactinemia [7-9].
Conclusion
PCOS is a major health threat in women population. It is determined with the various biochemical interactions between vital characters such as clinical, biochemical, hormonal and gynecological parameters as risk factors for PCOS. Our study indicated that BMI increase would be resulting in and waist/ hip ratio and might worsening the chances for PCOS. Future research will aid in identifying risk factors that contribute of PCOS, particularly long-term investigations with the objective of altering environmental variables to greatly lower risk.
References
- Homburg R. Polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol 2008; 22:261-74.
- Taieb J, Mathian B, Millot F, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography–mass spectrometry in sera from 116 men, women, and children. Clin Chem 2003; 49:1381-1395.
- Herold DA, Fitzgerald RL. Immunoassays for testosterone in women: Better than a guess?. Clin Chem 2003; 49:1250-1251.
- Plymate SR, Matej LA, Jones RE, et al. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metabol 1988; 67:460-464.
- Taylor AE, McCourt B, Martin KA, et al. Determinants of abnormal gonadotropin secretion in clinically defined women with polycystic ovary syndrome. J Clin Endocrinol Metabol 1997; 82:2248-2256.
- Plymate SR, Matej LA, Jones RE, et al. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metabol 1988; 67:460-464.
- Jayagopal V, Kilpatrick ES, Jennings PE, et al. The biological variation of testosterone and sex hormone-binding globulin (SHBG) in polycystic ovarian syndrome: Implications for SHBG as a surrogate marker of insulin resistance. J Clin Endocrinol Metabol 2003; 88:1528-1533.
- Nestler JE, Powers LP, Matt DW, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metabol 1991; 72:83-89.
- Weenen C, Laven JS, Von Bergh AR, et al. Anti‐Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Basic Sci Reprod Med 2004; 10:77-83.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Pavithra Ramakrishna, Kalpana Thalava and G Jayalakshmi*
Department of Obstetrics and Gynaecology, Sri Lakshmi Narayana Institute of Medical Sciences Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaReceived: 16-May-2022, Manuscript No. JRMDS-22-66696; , Pre QC No. JRMDS-22-66696 (PQ); Editor assigned: 18-May-2022, Pre QC No. JRMDS-22-66696 (PQ); Reviewed: 02-Jun-2022, QC No. JRMDS-22-66696; Revised: 07-Jun-2022, Manuscript No. JRMDS-22-66696 (R); Published: 14-Jun-2022
