Research - (2020) Volume 8, Issue 6
An In Vitro Study of the Antimicrobial and Antibiofilm Effects of Iron Oxide Nanoparticles against Different Oral and Dental Pathogenic Species Isolated from HIV/AIDS Patients
Salmeh Kalbassi1* and Fatemeh Ahmadi2
*Correspondence: Salmeh Kalbassi, Department of Orthodontics, Orthodontist in private practice, Qatar, Email:
Abstract
Introduction: Human oral species colonize the teeth, tongue, oral mucosa, hard palate, carious lesions and periodontal pocket. Nowadays, antibiotic resistance and invasive fungal infections has become a worldwide problem in public health and biofilm forming plays a key role in these issues.
Aim: In this study, the augmented use of Iron oxide nanoparticles and their status as emerging therapeutic applicants has motivated researchers to the evaluation of the antibacterial and antibiofilm effects of these nanoparticles against different oral and dental pathogenic species isolated from immunodeficient patients.
Materials and methods: A total of 12 immunodeficient patients’ samples were collected for this study using sterilized curettes. Microbial and fungal agents were isolated and identified using standard methods. Commercially obtained nanoparticles were examined using SEM and their size and structure were documented. Then, the multi-drug resistant strains were identified and the effects of IONPs on biofilm associated genes of these strains were evaluated using Real time PCR method.
Results: The age range of the participants was between 37 to 49 years with mean age of 43 ± 3.21 years. Finally, two multidrug resistant species of C. albicans and S. mutans were identified. RT-PCR examination showed down regulated ALS2 gene expression in C. albicans (-6.46-fold changes, p=0.007) and GtfB gene in S. mutans (-9.26-fold changes, p=0.001) as compared to a housekeeping gene upon exposure to NPs.
Conclusion: Iron oxide nanoparticles showed antibiofilm activities and effectively prevent multidrug resistant C. albicans and S. mutans strains to form biofilms. These findings suggest that IONPs could be embedded into matrices or materials used for fabrication of dentures, orthodontic appliances and dentistry devices to avoid colonization, adherence and biofilm formation of fungal and bacterial agents.
Keywords
HIV/AIDS patients, Oral, Dental, Nanoparticles.
Introduction
Bacterial and fungal pathogens can destroy the oral hygiene of a human being and impose huge medical burden. Thus, finding the elements that can remove or to minimize these pathogenic species is of top priority in respect to this issue [1,2]. In recent years, nanoparticles and probiotic bacteria attracted the attention of an increasing number of researchers from several disciplines due to their various application. Nanoparticles (NPs) have been existing on Earth for millions of years and have various properties including drug and gene delivery, detection of proteins, separation and purification of biological molecules and tissue engineering. NPs unique size-dependent physical properties make these agents essential in many aspects of human activity [3].
Biofilm is a cluster of microorganisms including bacteria and fungi attached to a surface (and/or to each other) and enclosed in extracellular polymeric substance (EPS) produced by themselves. Most of the biomass of the biofilm comprises hydrated EPS rather than microorganisms’ cells. Biofilm formation is a unique survival strategy of many microorganisms. This structure is a spongelike system that provides surfaces for the absorption of a wide range of molecules that can be sequestered from the microenvironment. In animal host biofilms are found in environments such as in or on the otolaryngologic, vaginal and gastrointestinal tracts. One of the first samples Anton van Leeuwenhoek, first scientists who detected bacteria with microscope in 1683, examined was his own dental plaque which is a biofilm [4,5].
Most common bacterial and fungal species isolated from infected areas in the mouth include Actinobacillus actinomycetemcomitans, Streptococcus mutans, Porphyromonas gingivalis, Prevotella intermedia, Bacteroides forsythus, Campylobacter rectus, Eubacterium species, Fusobacterium nucleatum, Eikenella corrodens, Peptostreptococcus micros and Candida albicans. More than 95% of these microorganisms can form biofilm [5,6]. Nowadays, development of multi drug resistant strains and complications or treatment failures following systemic and long-term use of antibiotics and antifungal medications have increased in immunodeficient patients [6]. In dentistry, using nanoparticles is one of the most effective methods to reducing bacterial adhesion and preventing biofilm formation that has been reported by previous investigations. Development of white spot lesion during orthodontic treatment is one of the most common problems during orthodontic therapy. In addition, large amount of data indicating that bacterial and fungal agents can growth in the adhesion area, bond between tooth enamel and orthodontic brackets [7,8]. A study evaluated the antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles and showed that a composite adhesive containing silver nanoparticles had found to be significantly lower cariogenic streptococci (Streptococcus mutans) adhesion to trial composite adhesives than routine orthodontic composite [9].
Lately, iron oxide nanoparticles (IONPs) have attracted much attention because of their ultrafine size, magnetic properties, and biocompatibility that are desirable for a wide range of biomedical applications and exhibit intrinsic qualities that enhance their ability to be used against pathogenic microorganisms [10,11].
An increasing frequency of systematic diseases, prolonged average life expectancies, the use antibiotics and immunosuppressive medications and the advent of the HIV have resulted in an increase in the number of immunocompromised patients within the community [12,13]. In this study, the augmented use of IONPs and their status as emerging therapeutic applicants has motivated researchers to the evaluation of the antibacterial and antibiofilm effects of these nanoparticles against different oral and dental pathogenic species isolated from immunodeficient patients.
Materials and Methods
Strains isolation
A total of 12 healthy and HIV negative individuals (control group) and 12 immunodeficient patients’ samples (case group) were collected for this study using sterilized curettes. In general, ten individuals with HIV/AIDS and two patients who underwent immunosuppressive therapy were studied. Patients with positive HIV test who had medical files and signed written consent to participate in the study were included as case group. Patients with incomplete medical files, who want to withdraw from study and individual with no written consent were excluded. All procedures involving human participants were performed in accordance with the 1964 Helsinki declaration and its later amendments (EN.9551621). The clinical bacterial strains and fungal species isolated from oral cavity of the participants with oropharyngeal Candidiasis (OCD). The samples were collected by scraping the subject’s oral mucosa and tongue with a sterile swab. All oral swabs were transported to the Pasargad Research Laboratory without any delay and prepared for laboratory investigations on the same day. The bacterial infections identified by the Gram staining, and microbiological standard methods. The samples subjected to falcon tubes containing 3 ml thioglycolate broth. Then, the tubes were centrifuged at 12,000 rpm for 10 min and the supernatant was discarded. The precipitate was suspended in 1 ml of phosphate-buffered saline to obtain a concentrated sample suspension. One loop full of concentrated suspension was inoculated onto EMB, Mitis Salivarius agar with potassium tellurite medium, MacConkey and CHROMagar Candida culture media using a standard streak plate method. All culture plates were incubated at 37°C for 24 h, and the growth of microorganism was observed as pink-and white-colored colonies. Definitive identification of isolates was performed through conventional biochemical tests) following 16SrRNA PCR using 27F (Forward primer 5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (Reverse primer 5’-GGTTACCTTGTTACGACTT-3’) primers, as described previously (10]. PCR products were subjected to 16SrRNA fragment sequencing. The relative molecular sizes of the PCR products were compared against the sequences from the Gen Bank database, National Centre for Biotechnology Information (NCBI) using Basic Local Alignment Search Tool (BLAST) analysis program.
Molecular identification
Genomic DNA of all isolates were extracted using (Qiagen, Germany) according to manufacturer instructions. The PCR reaction was performed in a final volume of 25 μl containing 6 μl template DNA (200 ng), 10 picomoles of forwarding primer (1 μl), 10 picomoles of reverse primers (1 μl), 4 μl of distilled water and 13 μl of Amplicon 2x master mix. The amplification conditions used were one cycle of initial denaturation at 94°C for 3 minutes, followed by 40 cycles of 95°C for 30 seconds, 54°C for 25 seconds, and 70°C for 30 seconds. The amplicons were separated by 1.2% agarose gel electrophoresis at 80 V for 2 h. After electrophoresis, fragments were stained by ethidium bromide and visualized using gel documentation apparatus.
Iron oxide nanoparticles preparation and characterization
Fe3O4 NPs were commercially obtained from Merck (Frankfurt, Germany). IONPs size and morphology were assessed with Scanning Electron Microscopy (SEM).
Determination of the antimicrobial and antifungal activity of IONPs
The antimicrobial and antibiofilm activity of NPs determined against multi-resistant isolates with strong biofilm formation ability. To recognize multi resistant isolates, the antibiotic susceptibility pattern of microorganisms was determined by modified Kirby Bauer disc diffusion method against the following antibiotics: penicillin-G (P) 10 units, ampicillin (AMP) 10μg, cefotaxime (CTX) 30μg, erythromycin (E) 15μg, cefazolin (CZ) 30μg, methicillin (MET) 5μg, lincomycin (L) 2μg, clindamycin (CC) 2μg and vancomycin V (30μg) (Merck, USA). The results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) methodology. Antifungal Susceptibility testing using Fluconazole (25μg), Nystatin (100 units), Ketoconazole (1μg), was used to identify the resistance strains. To assess the biofilm formation of multi-resistant microorganisms, micro titer plate and 0.1% crystal violet (CV) method was used as described by Fernandes et al. [14] and Beytollahi et al. [15], with slight modifications. Cultures were inoculated by adding 12 μL of an overnight culture of microorganisms into 1 ml of sterile media, and the tubes were incubated statically at either 37°C or at room temperature (~21°C) for two days. The supernatant was then discarded, and the adhered cells were rinsed three times with distilled water, and the tubes were patted dry on a paper towel. Two ml of a 0.1% CV solution was added to each tube to stain the adhered biomass and the tubes incubated for 26 min at 25°C. The CV dye was discarded, and the tubes were again rinsed three times with distilled water and were patted dry. Tubes were photographed to document the amount of biofilm that was adhered to the glass surface. The optical density of the wells was measured at 595 nm using a micro ELISA auto reader. To distinguish strong biofilm producers, an optical density of <0.120 for the non-biofilm producer, 0.120–0.240 for moderate biofilm producer and >0.240 was chosen. The IONPs were tested for antimicrobial activity by welldiffusion method against biofilm-producing strains.
The pure cultures of microorganism were subcultured onto sterile MHB/Sabouraud Dextrose Agar (SDA) and incubated at 37°C for overnight. After overnight culture of the broth, the yeasts were harvested in the late exponential growth phase, washed twice with 5 ml of phosphate-buffered saline (PBS; pH 7.2; Ca2+ and Mg2+ free), and suspended to 107 cells/ml by adjusting the optical density of the suspension to 0.38 at 520 nm. Wells of 6-mm diameter were made on MHA plates using gel puncture. Each strain was swabbed uniformly onto the individual plates via sterile cotton swabs. Using a micropipette, 100, 200 and 500 μg/ml of NPs were poured onto each of the three wells on all plates. Serial dilution of NPs was added to test wells. Following incubation at 32°C for 20 h, the different levels of inhibition zone were measured. All the tests were carried out in triplicate.
Quantitative real-time PCR
The expression levels of biofilm formation genes (Als1,2 for C. albicans [16] and GtfB, C for S. mutans [17]) were determined by quantitative real-time PCR (qRT-PCR). The primers used for qRT-PCR are in Table 1.
| Primer | Sequence 5´-3´ |
|---|---|
| ALS1 | Forward: 5´-GGCTACTCGGTTGATGTAG-3´ |
| Reverse: 5´-GGTACTGTTATAGATGTCGA-3 | |
| ALS2 | Forward: 5´-AGGTTCATCGGTTGATTTGG-3´ |
| Reverse: 5´-CCCATTGCACCAGATGTGTA-3 | |
| GtfB | Forward: 5´- ACTACACTTTCGGGTGGCTGG-3´ |
| Reverse: 5´- CAGTATAAGCGCCAGTTTCATC-3 | |
| GtfC | Forward: 5´- CTCAACCAACCGCCACTGTT-3´ |
| Reverse: 5´- GGTTTAACGTCAAAATTAGCTGTATTAGC-3 |
Table 1: Primers used for quantitative Real-Time PCR.
Briefly, the total bacterial RNA of isolates was extracted using a RNeasy Mini Kit (CinnaGen, Iran) and was quantified by spectrometry (NanoDrop, Thermo Scientific, USA). cDNA was then synthesized using a Prime Script RT Reagent Kit (CinnaGen, Iran) and was quantified using SYBR Green (Life Technologies). Ultimately, qRT-PCR was performed with a SYBR Premix Ex Taq II Kit (CinnaGen, Iran) on the thermocycler System (Eppendorf, Hamburg, Germany), with an initial incubation at 95ºC for 125 seconds, followed by 40 cycles of 15 s at 94ºC and 1 min at 62ºC. Relative expression of the target genes was obtained using beta actin housekeeping gene. The threshold cycle (CT) numbers were confirmed by the detection system software, and data were analyzed based on ΔΔCt method. The expression levels of target genes were specified and compared with each other. Each reaction was carried out in triplicate, and statistical analysis conducted via SPSS ver.16.
Statistical analysis
All data were analyzed using SPSS software (ver.19.122). Qualitative data described by frequency and percentage using Chi-square test and student t-test. Statistically, significant difference was considered when P<0.05.
Results
Isolated microorganisms
The age range of the participants was between 37 to 49 years with mean age of 43 ± 3.21 years. In general manner, different microorganisms including two candida species (candida albicans and candida tropicalis) and 12 Streptococcus mutans strains had isolated from patients’ lesions and dental cavities. No microorganisms were isolated from the samples of apparently healthy individuals. Patients showed oral hairy leukoplakia (66.66%), oral candidiasis (33.33%), herpes simplex virus infection (25%), recurrent aphthous ulcers (16.66%) and linear gingival erythema (16.66%) (Figure 1).
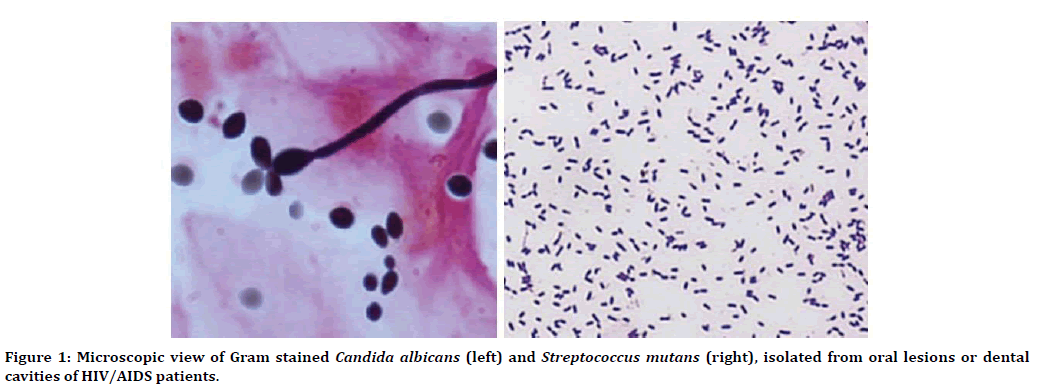
Figure 1. Microscopic view of Gram stained Candida albicans (left) and Streptococcus mutans (right), isolated from oral lesions or dental cavities of HIV/AIDS patients.
C. albicans strain was resistant to fluconazole but susceptible to Nystatin and Ketoconazole. However, C. tropicalis strain was completely susceptible to Nystatin, Fluconazole and Ketoconazole. On the other hand, all S. mutans strains showed resistance to Ampicillin (69%) and Cefotaxime (61%). Penicillin, methicillin, and vancomycin were the most effective antibiotics against S. mutans isolates and resistance rate do not exceed 8.3%. Two microorganisms, one C. albicans and one S. mutans (sm19), among all isolated strains had showed multi-resistant pattern to antibiotic and antifungal agents. The biofilm formation ability of these multidrug-resistant microorganisms was assessed using the well diffusion method and indicated that both isolated can form fully established biofilms. C. tropicalis did not produce any biofilm.
Nanoparticle identification
SEM images of obtained iron oxide nanoparticles are shown below: The particle size range was from 19 to 33.52 nm. The IONPs were mainly spherical and approximately monodisperse (Figure 2).
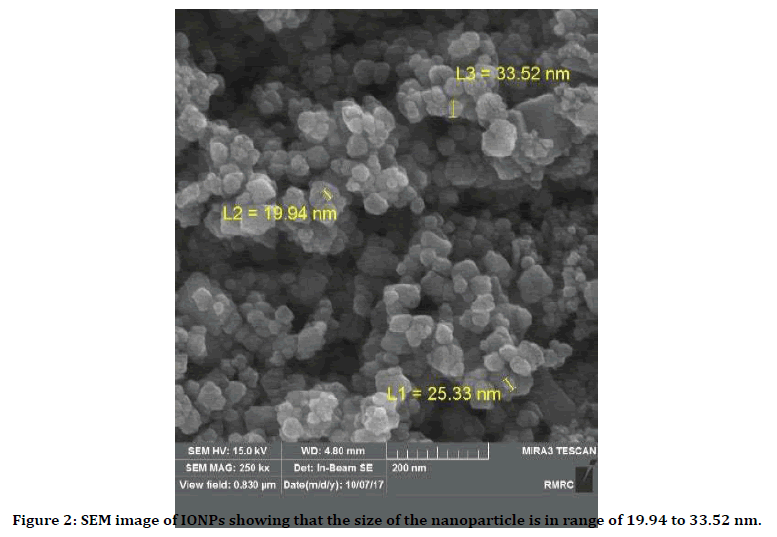
Figure 2. SEM image of IONPs showing that the size of the nanoparticle is in range of 19.94 to 33.52 nm.
IONPs effects on microorganisms
The IONPs inhibited growth in both multidrugresistant isolates of S. mutans and C. albicans according to the CLSI. In this study, Minimum fungicidal concentrations (MFC) and minimum inhibitory concentration (MIC) values of IONPs were 50 to 200 μl/ml and 128 μg/ml, respectively. IONPs inhibited C. albicans and S. mutans isolates to form a biofilm at 256 μg/ml and 128 μg/ml concentrations, respectively. We examined the effects of NPs on gene expression, which may reveal the source for the inhibited biofilm formation.
IONPs effects on gene expression
We examined the effects of IONPs on biofilm producing genes expression, which may reveal the source for the inhibited biofilm formation. Genes encoding for biofilm production were explored using real-time polymerase chain reaction (RT-PCR) following a 24 h exposure to varying concentrations of NPs. Comparison of ALS2 gene expression in C. albicans untreated and treated with NPs showed average +0.011- and -6.46-fold changes (Confidence Level=95%, p=0.346 and p=0.007, respectively). As shown in figure below (Figure 3), relative mean fold changes of the GtfB gene as compared to a housekeeping gene upon exposure to NPs was -9.26 fold changes (CI=95%, p=0.0001). There was no significant difference between ALS1 and GtfC in strains and control gene.
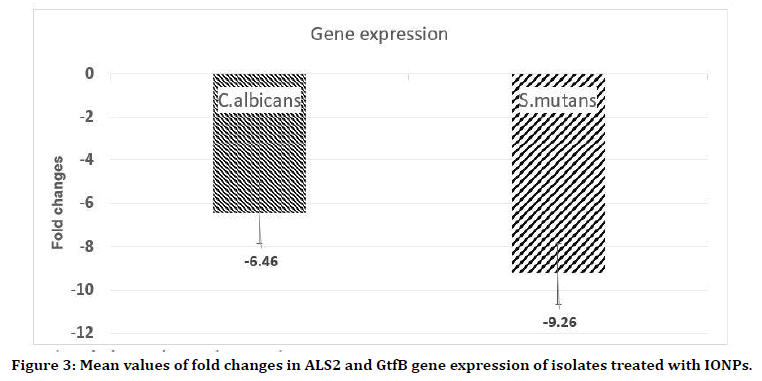
Figure 3. Mean values of fold changes in ALS2 and GtfB gene expression of isolates treated with IONPs.
Discussion
There are over 900 different bacterial and fungal species in the oral microflora. Human oral cavity microorganisms are responsible for the two most common oral and dental diseases in humans like dental caries and periodontal disease. Those species colonize the teeth, tongue, oral mucosa, hard palate, carious lesions, and periodontal pocket. Nowadays, antibiotic resistance and invasive fungal infections has become a worldwide problem in public health and biofilm forming plays a key role in these issues [4,5,8]. Both fungal and bacterial agents can form biofilms to protect themselves from a challenging environment with several host defense mechanisms and antibiotics. Surveys of NPs as antibacterial, antifouling, and antibiofilm agents have increased in recent years due to antibiotic resistance issue [4,5,8]. This in vitro study aimed to evaluation of the antibacterial and antibiofilm effects of IONPs against different oral and dental multi drug resistant pathogenic species isolated from HIV/AIDS patients. Our group isolated two multidrug species of C. albicans and S. mutans. C. albicans strain was resistant to fluconazole while S. mutans strains showed resistance to Ampicillin and Cefotaxime.
Bacterial and fungal resistance to antibiotics and antifungal agents such as ampicillin and fluconazole is a health issue in numerous parts of the world. In our study we observed a highly significant level of Ampicillin (69%) and Cefotaxime (61%) in HIV-patients S. mutans clinical isolates. The high prevalence of resistance to beta lactams in S. mutans in our study was higher than that previously observed in some studies in south Africa, Yemen and Spain in oral isolated streptococcus spp. [18-20] which can be attributed to sample size, isolated strains and patients underlying diseases. Updated evidence on antibiotic susceptibility testing such as reported in our study helps scientists to design new strategies for operative prophylaxis against oral and dental infections, especially in HIV-infected individuals.
Salari et al. in a similar survey, studied molecular mechanisms of azole resistance in 20 fluconazole-resistant C. albicans isolates obtained from Iranian HIV-infected patients with oropharyngeal candidiasis and showed that the overexpression of the CDR1 gene correlated strongly with increasing resistance to fluconazole (P<0.05) [21]. Our study showed that the IONPs can downregulate the biofilm construction associated genes in dose dependent manner and results to interference and inhibition in biofilm formation in both S. mutans and C. albicans. Another study by Seddighi et al, about antifungal effect of IONPs against different Candida species showed that C. tropicalis, C. albicans and C. glabrata spp. were most susceptible to IONPs. In addition, their finding, in line with our data, showed that the IONPs possessed antifungal potential against pathogenic Candida spp. and could inhibit the growth of all the tested Candida spp [22].
Pérez-Díaz et al. assessed silver nanoparticles antimicrobial activity against S. mutans, and showed that these nanoparticles effectively inhibited the growth of a planktonic S. mutans clinical isolate and killed established S. mutans biofilms, which suggests that AgNPs could be used for prevention and treatment of dental caries. These findings are in line with our data about effectiveness of the nanoparticles in antimicrobial activities [23].
There is a huge amount of data on the bactericidal and fungicidal activity mechanism of IONPs, suggesting that oxidative damage is the primary mechanism of the antimicrobial effect of IONPs [3,10]; in the present study, the changes in the ALS and GtfB genes expression levels can be considered as yet another effective anti-biofilm mechanism associated with these agents.
Conclusion
Iron oxide nanoparticles showed bactericidal and fungicidal activities and effectively prevent multidrug resistant C. albicans and S. mutans strains to form biofilms. These findings suggest that IONPs could be embedded into matrices or materials used for fabrication of dentures, orthodontic appliances, and dentistry devices to avoid colonization, adherence, and biofilm formation of fungal and bacterial agents.
References
- Dehghani R, Sharif MR, Moniri R, et al. The identification of bacterial flora in oral cavity of snakes. Comparative Clin Pathol 2016; 25:279-283.
- Baker JL, Bor B, Agnello M, et al. Ecology of the oral microbiome: beyond bacteria. Trends Microbiol 2017; 25:362-374.
- Dadfar SM, Roemhild K, Drude NI, et al. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv Drug Delivery Reviews 2019; 138:302-325.
- Chevalier M, Ranque S, Prêcheur I. Oral fungal-bacterial biofilm models in vitro: a review. Med Mycol 2018; 56:653-657.
- Mosaddad SA, Tahmasebi E, Yazdanian A, et al. Oral microbial biofilms: An update. Eur J Clin Microbiol Infectious Dis 2019; 38:1-15.
- Lasserre JF, Brecx MC, Toma S. Oral microbes, biofilms and their role in periodontal and peri-implant diseases. Materials 2018; 11:1802.
- Xia Y, Chen H, Zhang F, et al. Injectable calcium phosphate scaffold with iron oxide nanoparticles to enhance osteogenesis via dental pulp stem cells. Artificial Cells Nanomed Biotechnol 2018; 46:423-433.
- Priyadarsini S, Mukherjee S, Mishra M. Nanoparticles used in dentistry: A review. J Oral Biol Craniofacial Res 2018; 8:58-67.
- Ahn SJ, Lee SJ, Kook JK, et al. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Materials 2009; 25:206-213.
- Tong S, Quinto CA, Zhang L, et al. Size-dependent heating of magnetic iron oxide nanoparticles. Acs Nano 2017; 11:6808-6816.
- Li J, Nickel R, Wu J, et al. A new tool to attack biofilms: driving magnetic iron-oxide nanoparticles to disrupt the matrix. Nanoscale 2019; 11:6905-6915.
- Khedri S, Santos A, Roudbary M, et al. Iranian HIV/AIDS patients with oropharyngeal candidiasis: identification, prevalence and antifungal susceptibility of Candida species. Letters Applied Microbiol 2018; 67:392-399.
- Frimpong P, Amponsah EK, Abebrese J, et al. Oral manifestations and their correlation to baseline CD4 count of HIV/AIDS patients in Ghana. J Korean Association Oral and Maxillofacial Surg 2017; 43:29-36.
- Fernandes RA, Monteiro DR, Arias LS, et al. Biofilm formation by Candida albicans and Streptococcus mutans in the presence of farnesol: A quantitative evaluation. Biofouling 2016; 32:329-338.
- Beytollahi L, Pourhajibagher M, Chiniforush N, et al. The efficacy of photodynamic and photothermal therapy on biofilm formation of Streptococcus mutans: an in vitro study. Photodiagnosis Photodynamic Therapy 2017; 17:56-60.
- Nobile CJ, Schneider HA, Nett JE, et al. Complementary adhesin function in C. albicans biofilm formation. Current Biol 2008; 18:1017-1024.
- Senadheera MD, Lee AW, Hung DC, et al. The Streptococcus mutans vicX gene product modulates gtfB/C expression, biofilm formation, genetic competence, and oxidative stress tolerance. J Bacteriol 2007; 189:1451-1458.
- Al-Shami IZ, Al-Hamzi MA, Al-Shamahy HA, et al. Efficacy of some antibiotics against Streptococcus mutans associated with tooth decay in children and their mothers. On J Dent and Oral Health 2019; 2.
- Sounah SA, Madfa AA. Correlation between dental caries experience and the level of Streptococcus mutans and lactobacilli in saliva and carious teeth in a Yemeni adult population. BMC Res Notes 2020; 13:1-6.
- Pereira CA, Romeiro RL, Costa ACBP, et al. Susceptibility of Candida albicans, Staphylococcus aureus, and Streptococcus mutans biofilms to photodynamic inactivation: An in vitro study. Lasers Med Sci 2011; 26:341-348.
- Salari S, Khosravi A, Mousavi S, et al. Mechanisms of resistance to fluconazole in Candida albicans clinical isolates from Iranian HIV-infected patients with oropharyngeal candidiasis. J Mycol Med 2016; 26:35-41.
- Seddighi NS, Salari S, Izadi AR. Evaluation of antifungal effect of iron-oxide nanoparticles against different Candida species. Nanobiotechnol 2017; 11:883-888.
- Pérez-Díaz MA, Boegli L, James G, et al. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Materials Sci Engineering 2015; 55:360-366.
Author Info
Salmeh Kalbassi1* and Fatemeh Ahmadi2
1Department of Orthodontics, Orthodontist in private practice, Iran (Tehran, Isfahan), Oman (Muscat), UAE (Dubai) and Qatar, Qatar2Faculty of Biological Sciences, Tehran-North Branch, Islamic Azad University, Tehran, Iran
Citation: Salmeh Kalbassi, Fatemeh Ahmadi, An In Vitro Study of the Antimicrobial and Antibiofilm Effects of Iron Oxide Nanoparticles Against Different Oral and Dental Pathogenic Species Isolated from HIV/AIDS Patients, J Res Med Dent Sci, 2020, 8 (6): 34-40.
Received: 24-Jul-2020 Accepted: 10-Sep-2020
