Research - (2022) Volume 10, Issue 1
Anticancer Effect of Cymodocea Serrulata Sea grass Crude Extract against Breast Cancer Cell Line
Nandini Palanivel, P Sivaperumal*, D Ezhilarasan, Elumalai and Lakshmi T
*Correspondence: P Sivaperumal, Department of Pharmacology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chenna, India, Email:
Abstract
Introduction: Breast cancer is cancerous cells which develop in breast cells. The cancer forms in either the lobules or the ducts of the breast. Cancer can also occur in the fatty tissue or the fibrous connective tissue within your breast. Cymodocea serrulata (Family Cymodoceaceae) Material and Methods: The sea grass Cymodocea serrulata was collected from Mimisal, Tamilnadu. The sea grass was washed thoroughly with tap water then shade dried on table tissue paper for 3 weeks and turned into a fine powder and then used for the research. Results: The crude extract obtained from Cymodocea serrulata shows potential anticancer activities. Analysis of cell morphology changes by a phase contrast microscope was done. 3 X 104 cells were seeded in 6 well plates and treated (concentration for MCF-7 cells) for 24h. At the end of the incubation period, the medium was removed and cells were washed once with a phosphate buffer saline (PBS pH 7.4). The plates were observed under a phase contrast microscope. Conclusion: The anticancer effect of Cymodocea serrulata crude extract against breast cancer cell line is found. Therefore, many people have turned to traditional medicines that are considered more safe and economical, because they use natural ingredients. Natural ingredients contain several active compounds which give pharmacological effects. In general, those active compounds are secondary metabolites.
Keywords
Anticancer, Sea grass, Breast cancer cell line, Cymodocea serrulata
Introduction
Sea grass is a plant that lives in tidal areas, possesses unique secondary metabolites, which have roles as anticancer bioactive compounds. The method used in the experimental method which continues the extraction towards sea grass leaves using n-hexane (non-polar), ethyl acetate (semi polar) and ethanol (polar) [1]. Extract obtained was analyzed for its anticancer activity by using some cells. Breast cell lethality by semi polar extract, polar extract and doxorubicin are 48.11%, 15.32 % and 48.75%, respectively. Ethyl acetic crude extract of fresh sea grass contains phytochemical compounds of alkaloid, terpenoids, polyphenol and flavonoid. Cancer disease has been a major health problem in the world and also in Indonesia. Based on WHO data on 2013, cancer incidence increased from 12.7 million cases (2008) to 14.1 million cases (2012). It is predicted that in 2030, cancer incidence could reach up to 26 million people, in which 17 million people could die because of cancer, particularly in underdeveloped countries and developing countries [2,3].
Several treatments to prevent and cure cancer have been done, such as radiotherapy, chemotherapy, or using synthetic medicine. In general, anticancer therapy has been felt to be sufficient to give good results, but it has side effects and the costs are quite. Radiotherapy and chemotherapy have limitations [4]. The effectiveness of rays used for radiotherapy will decrease along with the increase of tumor size, meanwhile the increase of dosage given to the level exceeds its toxicity level will give effect to normal human tissues and organs. The use of chemical drugs such as chemotherapy kill not only the tumor cells but also damage blood cells, which causes the decrease in immunity or even death, resulting from complications due to side effects of drugs. Several cervical cancer cells have become resistant towards treatment using radiation and chemotherapy [4,5]. Therefore, many people have turned to traditional medicines that are considered more safe and economical, because they use natural ingredients. Natural ingredients contain several active compounds which give pharmacological effects. In general, those active compounds are secondary metabolites. Secondary metabolites have been known as sources of medical therapy, for example as antibacterial and anticancer medicines, etc. Majority of secondary metabolites are synthesized by organisms to adapt to their environment, thus, the search of secondary metabolites that are able to act as anticancer bioactive mainly focused on organisms that live in extreme environmental condition. One of the extreme environments is in a tidal area. Organisms which live in the tidal area and have been reported to contain anticancer bioactive compounds are sponges and their entophytic microbes, Ascidia (sea squirt) [6]. Microbes that associated with sea biota, such as marine derived fungi, mangroves and their entophytic microbes, and sea grass and its endophytic microbes [7]. Symbiotic or epiphytic bacteria on sea grass, a term for bacterial colony that lives, grows and associates with sea grass, has been reported to contain similar bioactive compounds to its symbiotic. Sea grass is a hydrophyte that lives in tropical or subtropical tidal coastal areas. Around the world, there are 52 types of sea grass, 15 of which are present in Indonesia. Several researches showed that sea grass has potential as antioxidant, anticancer and antibacterial agents. Compounds in sea grass that are suspected to be able to inhibit cancer cell proliferation and inactivate pathogenic bacteria are flavonoid, saponin, steroid, terpenes, tannin and alkaloid [8]. It is stated that sea grass Enhalus acoroides contains stigmasterol, sitosterol and alkaloid. Sea grass Thalassia testudinium contains glycosides and phenol compounds which are potential to act as antifungal and anticancer, while seagrass Halodule uninervis contains steroid compounds which acts as antibacterial and anticancer [2,3,9,10]. It have stated that crude extract from seagrass Thalassia hemprichii,Cymodocea serrulata acoroides had high phenolic content. Moreover, reported the cytotoxicity of crude extract from sea grass [11]. Enhalus acoroides Cymodocea serrulata toward Artemia salina concentration of 404.88 ppm and 136.398 ppm, respectively. The highest phenolic content is on the leaves part. One of seagrass that can be found in tidal coastal areas in Indonesia is Cymodocea serrulata [11,12]. Our team has extensive knowledge and research experience that has translated into high quality publications [13-25]. The types of sea grass were reported to contain anticancer bioactive compounds, another research to determine the potential of sea grass and Cymodocea serrulata as a source of anticancer bioactive compounds should also be conducted. Therefore, the aim of this research was to determine the potential of sea grass Cymodocea serrulata fresh leaves extract as an anticancer agent.
Materials and Methods
Preparation of Extract
A 10g of dried powdered sea grass samples were mixed with 100ml of methanol/Ethanol (V/V) and allowed to place for 24 hours at ambient temperature. Then the mixture was passing through what man filter paper (No. 4) then the filtrate was centrifuged at 3000rpm for 10min and further filtered by 0.45μm syringe micro filter. At last, the solvents are evaporated via a vacuum rotary evaporator until samples obtain in powder form. Then the sample was stored in a shadowy aluminium container at 4ºC for further analysis.
MTT Assay
The proliferation of MCF-7 cells was assessed by MTT assay. MCF-7 cells were plated in 48 well plates at a concentration of 2x10 4 cells/well 24 hours after plating, cells were washed twice with 500μl of serum-free medium and starved by incubating the cells in serum-free medium for 3 hours at 37ºC. After starvation, cells were treated with sea grass extract in different concentrations for 24 hours. At the end of treatment, the medium from control and Sea grass extract treated cells were discarded and 200μl of MTT containing DMEM (0.5mg/ml) was added to each well. The cells were then incubated for 4h at 37ºC in the CO2 incubator.
The MTT containing medium was then discarded and the cells were washed with 1x PBS. The crystals were then dissolved by adding 200μl of solubilisation solution and this was mixed properly by pipetting up and down. Then the formed crystals were dissolved in dimethylsulfoxide (200μl) and incubated in dark for an hour. Then the intensity of the colour developed was assayed using a Micro ELISA plate reader at 570 nm. The number of viable cells was expressed as the percentage of control cells cultured in serum-free medium. Cell viability in the control medium without any treatment was represented as 100%. The cell viability is calculated using the formula: % cell viability=[A570 nm of treated cells/A570 nm of control cells]×100.
Results and Discussion
The crude extract obtained from Cymodocea serrulata showed potential anticancer activities against Breast cancer cell lines. The cancer forms in either the lobules or the ducts of the breast. Cancer can also occur in the fatty tissue or the fibrous connective tissue within your breast. Cymodocea serrulata (Family Cymodoceaceae).

Figure 1: The picture represent the sea grass Cymodocea serrulata present under the sea.

Figure 2:The picture represents the single plant of the Cymodocea serrulata.

Figure 3:The picture represents the dried sea grass samples.

Figure 4:The picture represents the crude extracts.
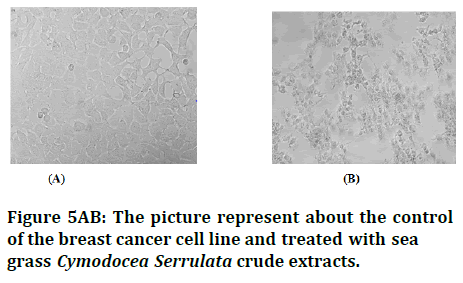
Figure 5AB:5AB: The picture represent about the control of the breast cancer cell line and treated with sea grass Cymodocea Serrulata crude extracts.
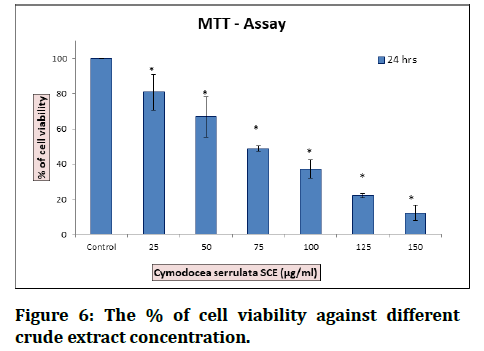
Figure 6:The % of cell viability against different crude extract concentration.
The Results showed that non-polar extract has no anticancer activity, while semi polar and polar extract showed their potential as an anticancer agent. Breast cell lethality level by semi polar extract was higher than polar extract, but not significantly different with cancer medicine doxorubicin [2]. The extract of C. serrulata reveals the presence of phytocompounds that are biologically active. According to the chromatogram obtained by GCMS ethanol extract of C. serrulata consists of palmitic acid, myristic acid, and pentadecanoic acid as a major component. They may be produced by the plant defenses itself from stress as secondary metabolites. These cytoprotectants proved to possess pharmacological activity in a similar way as synthetic drugs. The palmitic acid reported possessing anticancer activity, antimicrobial, and nematicide activity. The palmitic acid increases the number of probiotic bacteria in the gut; thus, they are involved in the development of the intestine. It is required in the biosynthesis of lung lecithin, which is related to fetal maturation as well as it has been reported that presence of palmitic acid in the Nigerian meal can partly be related to the low incidence of respiratory disease. Palmitic acid reported inhibiting human hepatoma cell growth in a dose dependent and time-dependent manner. Thus, they possess anticancer. One of seagrass that can be found in tidal coastal areas in Indonesia is Cymodocea serrulata. Since other types of seagrass were reported to contain anticancer bioactive compounds, another research to determine the potential of seagrass Cymodocea serrulata as a source of anticancer bioactive compounds should also be conducted [26].
Cymodocea serrulata had high phenolic content. Moreover, reported the cytotoxicity of crude extract from seagrass Enhalus acoroides Cymodocea serrulata toward Artemia salina concentration of 404.88 ppm and 136.398 ppm, respectively.it is stated that the highest phenolic content is on the leaves part. Sea grasses were known to possess remarkable bioactivities. Cymodocea serrulata exhibited more potent antioxidant activity than ascorbic acid, Gallic acid. Another finding of seaweed Gracilaria tenuistipitata was antioxidant inactive. The water extracts of seaweed Gracilaria tenuistipitata were proved to contain bioactive compounds such as phenolic, flavonoid, and ascorbic acid, however their DPPH free radical scavenging activity was relatively weak, with 63.37% DPPH free radicals scavenged by 4 mg/ml-1 extract [27]. From the test results of cytotoxic, antimicrobial and antioxidant activities of 57 seaweed and sea grass extracts in this study, it could be concluded that samples extracted from closely taxonomic species were not completely homogeneous in biological activities. The reason may result from the divergence in geographic distribution, ages of seaweed and sea grass samples. We can further study using more volume of samples and this can be done as an antimicrobial and anti-cytotoxicity study against breast cancer cell line in future. WOS cite [28-44]
Conclusion
The anticancer effect of Cymodocea serrulata crude extract against breast cancer cell line is found. Therefore, many people have turned to traditional medicines that are considered more safe and economical, because they use natural ingredients. Natural ingredients contain several active compounds which give pharmacological effects. In general, those active compounds are secondary metabolites. Secondary metabolites have been known as sources of medical therapy, for example as antibacterial and anticancer medicines, etc.
Acknowledgement
We would like to thank Saveetha Dental College for providing the opportunity to conduct this study.
Conflict of Interest
There is no conflict of interest.
Source of Funding
Saveetha Dental College and Hospital, Chennai and Palanivel group of companies, Dindigul, Tamil Nadu.
References
- Priya RA, Saravanan K, Umarani B. Gas chromatography-mass spectrometry analysis and in vitro anticancer activity of Tectona grandis bark extract against human breast cancer cell line (mcf-7). Phyto chem Apple Acad Press 2018; 12:571-586.
- Gnanambal KM, Patterson J, Patterson EJ. Isolation of a novel antibacterial phenyl thioketone from the seagrass, Cymodocea serrulata. Phytotherapy Res 2015; 29:554-60.
- Santhanam R, Santhanam R, Suleria H. Biology and ecology of pharmaceutical marine plants. CRC Press 2018; 13.
- Vijayakumar S, Bhuvaneshwari V, Sumathi A. Antioxidant and anticancer potential of methanolic leaf extract of Moringa concanensis nimmo against human breast cancer cell line MCF-7. Intern J Pharm and Phytochem Res 2017; 9:750-4.
- Wawruszak A, Czerwonka A, Okla K, Rzeski W. Anticancer effect of ethanol Lycium barbarum (Goji berry) extracts on human breast cancer T47D cell line. Nat prod res 2016; 30:1993-6.
- S KC, Kalpana CS, Kalaivani P, et al. Phytochemical screening and effect of Cymodocea serrulata on HepG2-Human hepatocellular carcinoma cell line. Int J Res Pharm Sci 2020; 1684â??1691.
- Salehzadeh A, Bejarbaneh M, Jalali A. Cobalt oxide nanoparticle conjugated with thiosemicarbazide shows the anticancer activity against breast cancer cell line (T47D). Breast 2019; 1:48-72.
- Kalpana CS, Kalaivani P, et al. Deciphering the effect of hydro-alcoholic extract of Cymodocea serrulata on the cell cycle arrest and apoptosis. Int J Res Pharm Sci 2020; 3261â??71.
- De Azevedo JL, Quecine MC, editors. Diversity and benefits of microorganisms from the tropics. Springer 2017; 10.
- Kim SK, editor. Handbook of marine microalgae: Biotechnology advances. Academic Press 2015; 30.
- Ravikumar S, Syed Ali M, Anandh P, et al. Antibacterial activity of Cymodocea serrulata root extract against chosen poultry pathogens. Ind J Sci Technol 2011; 4:98-100.
- RathnaKumari P, Kolanchinathan P, Siva D, et al. Antibacterial efficacy of seagrass Cymodocea serrulata-engineered silver nanoparticles against prawn pathogen Vibrio parahaemolyticus and its combative effect on the marine shrimp Penaeus monodon. Aquaculture 2018; 493:158-64.
- Priyadharsini JV, Girija AS, Paramasivam A. In silico analysis of virulence genes in an emerging dental pathogen A. baumannii and related species Archives of oral biol 2018; 94:93-8.
- Vijayashree Priyadharsini J. In silico validation of the non-antibiotic drugs acetaminophen and ibuprofen as antibacterial agents against red complex pathogens. J periodontol 2019; 90:1441-1448.
- Paramasivam A, Priyadharsini JV, Raghunandhakumar S. N6-adenosine methylation (m6A): A promising new molecular target in hypertension and cardiovascular diseases. Hypertension Res 2020; 43:153-154.
- Priyadharsini JV, Girija AS, Paramasivam A. An insight into the emergence of Acinetobacter baumannii as an oro-dental pathogen and its drug resistance gene profileâ??An in silico approach. Heliyon 2018; 4:01051.
- Paramasivam A, Priyadharsini JV. Novel insights into m6A modification in circular RNA and implications for immunity. Cellular Mol Immunol 2020; 17:668-669.
- Paramasivam A, Priyadharsini JV, Raghunandhakumar S. Implications of m6A modification in autoimmune disorders. Cellular Mol Immunol 2020; 17:550-551.
- Girija AS, Shankar EM, Larsson M. Could SARS-CoV-2-induced hyper inflammation magnify the severity of coronavirus disease (CoViD-19) leading to acute respiratory distress syndrome? Frontiers Immunol 2020; 11:1206.
- Jayaseelan VP, Arumugam P. Exosomal microRNAs as a promising theragnostic tool for essential hypertension. Hypertension Res 2020; 43:74-75.
- Ushanthika T, Smiline Girija AS, Paramasivam A, et al. An in silico approach towards identification of virulence factors in red complex pathogens targeted by reserpine. Nat Prod Res 2021; 35:1893-1898.
- Ramalingam AK, Selvi SGA, Jayaseelan VP. Targeting prolyl tripeptidyl peptidase from Porphyromonas gingivalis with the bioactive compounds from Rosmarinus officinalis. Asian Biomed 2019; 13:197â??203.
- Kumar SP, GIRIJA AS, Priyadharsini JV. Targeting NM23-H1-mediated inhibition of tumour metastasis in viral hepatitis with bioactive compounds from ganoderma lucidum: A computational study. Ind J Pharm Sci 2020; 82:300-305.
- Mathivadani V, Smiline AS, Priyadharsini JV. Targeting epstein-barr virus nuclear antigen 1 (ebna-1) with murraya koengii bio-compounds: An in-silico approach. Acta virol 2020; 64:93-9.
- Girija AS. Fox3+ CD25+ CD4+ T regulatory cells (Tregs) may transform the n-CoV's final destiny to CNS! J Med Virol 2020.
- Rosalina D, Herawati EY, Musa M, et al. Lead accumulation and its histological impact on Cymodocea serrulata seagrass in the laboratory. Sains Malaysiana 2019; 48:813-22.
- Yang JI, Yeh CC, Lee JC, et al. Aqueous extracts of the edible Gracilaria tenuistipitata are protective against H2O2-induced DNA damage, growth inhibition, and cell cycle arrest. Mol 2012; 17:7241-54.
- Rajeshkumar S, Ezhilarasan D, Puyathron N, et al. Role of super magnetic nanoparticles in alzheimer disease. In Nano biotechnol Neurodegenerative Dis 2019; 225-240.
- Rajeshkumar S, Lakshmi T, Tharani M, et al. Green synthesis of gold nanoparticles using pomegranate peel extract and its antioxidant and anticancer activity against liver cancer cell line.
- Rajeshkumar S, Tharani M, Sivaperumal P, et al. Synthesis of Antimicrobial silver nanoparticles by using flower of Calotropis gigantea. J Complementary Med Res 2020; 11:8-16.
- Lakshmi T, Ezhilarasan D, Nagaich U, et al. Acacia catechu ethanolic seed extract triggers apoptosis of SCC-25 cells. Pharmacog Mag 2017; 405.
- Happy A, Soumya M, Kumar SV,et al. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem Biophys Rep 2019; 17:208-11.
- Rajeshkumar S, Sivaperumal P, Tharani M. Green Synthesis of zinc oxide nanoparticles by Cardiospermum. J Complementary Med Res 2020; 11:128-36.
- Rajeshkumar S, Tharani M, Sivaperumal P. Green Synthesis of selenium nanoparticles using black tea (Camellia Sinensis) and its antioxidant and antimicrobial activity. J Complementary Med Res 2020; 11:75-82.
- Jagadheeswari RJ, Lakshmi TL, Balusamy SR, et al. Biosynthesis of silver nanoparticles using Withania somnifera (L.) dunal extract and its antibacterial activity against food pathogens. Ann Phytomed 2020; 9.
- Roy A, Rajagopal P, Thangavelu L. Molecular docking analysis of compounds from Lycopersicon esculentum with the insulin receptor to combat type 2 diabetes. Bioinformation 2020; 16:748-52.
- Ramamoorthy K, Subramanian Raghunandhakumar RS, Paramasivam A, et al. Anticancer effects and lysosomal acidification in A549 cells by Astaxanthin from Haematococcus lacustris. Bioinformation 2020; 16:965.
- Akshayaa L, Lakshmi T, Devaraj E, et al. Data on known anti-virals in combating CoVid-19. Bioinformation 2020; 16:878.
- Rajeshkumar S, Agarwal H, Sivaperumal P, et al. Antimicrobial, anti-inflammatory and anticancer potential of Microbes mediated zinc oxide nanoparticles. J Complementary Med Res 2020; 11:41-8.
- Thangavelu L, Balusamy SR, Shanmugam R, et al. Evaluation of the sub-acute toxicity of Acacia catechu willd seed extract in a wistar albino rat model. Reg Toxicol Pharm 2020; 113:104640.
- Jackson K, Devaraj E, Lakshmi T, et al. Cytotoxic potentials of silibinin assisted silver nanoparticles on human colorectal HT-29 cancer cells. Bioinformation 2020; 16:817.
- Shaker Ardakani L, Surendar A, Thangavelu L, et al. Silver nanoparticles (Ag NPs) as catalyst in chemical reactions. Syn Commun 2021; 51:1516-36.
- Hashim IM, Ghazi IF, Kuzichkin OR, et al. Effects of primary stored energy on relaxation behavior of high entropy bulk metallic glasses under compressive elastostatic loading. Transactions Indian Institute Metals 2021; 14:1-7.
- Krishnan V, Lakshmi T. Bioglass: A novel biocompatible innovation. J adv pharm Technol Res 2013; 4:78.
- Hardoko PD, Yuli E. Anticancer potential of seagrass leaves Cymodecea serrulata Crude extract on HeLa cell. J Chem Pharm Res 2016; 8:571-6.
- Kannan Rengasamy RR, Rajasekaran A, Micheline GD, et al. Antioxidant activity of seagrasses of the Mandapam coast, India. Pharm Biol 2012; 50:182-7.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref          Â
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Nandini Palanivel, P Sivaperumal*, D Ezhilarasan, Elumalai and Lakshmi T
Department of Pharmacology, Saveetha Dental College and Hospital, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chenna, IndiaCitation: Nandini Palanivel, P Sivaperumal, D Ezhilarasan, Elumalai, Lakshmi T, Anticancer Effect of Cymodocea Serrulata Sea grass Crude Extract against Breast Cancer Cell Line , J Res Med Dent Sci, 2022, 10(1): 13-18
Received: 14-Dec-2021, Manuscript No. JRMDS-21-44650; , Pre QC No. JRMDS-21-44650 (PQ); Editor assigned: 16-Dec-2021, Pre QC No. JRMDS-21-44650 (PQ); Reviewed: 30-Dec-2021, QC No. JRMDS-21-44650; Revised: 04-Jan-2022, Manuscript No. JRMDS-22-44650 ; Published: 11-Jan-2022
