Research - (2022) Volume 10, Issue 1
Anti-Inflammatory Activity of Centella Asiatica Mediated Silver Nanoparticles
S Ganesh1, Abirami Arthanari1* and S Rajeshkumar2
*Correspondence: Abirami Arthanari, Department of Forensic Odontology, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Saveetha University, India, Email:
Abstract
Introduction: Inflammation is the body's main reaction to an illness or injury, and it is vital to our immunity. Because of the increasing prevalence of pain and pain-related disorders, as well as other health treatment complexities, it is important to be mindful of the anti-inflammatory medications available on the market. Furthermore, nonsteroidal anti-inflammatory medications have been related to side effects including stomach pain, heartburn, and ulcers. So, this study deals with the anti-inflammatory activity of Centella mediated silver nanoparticles Materials and methods: Collection of Centella asiatica extract then 1 g of Centella asiatica, leaf powder was dissolved in distilled water and boiled for 5–10 min at 60–70C. The solutions were then filtered using Whatman No. 1 filter paper. The filtered extract was collected, synthesis of silver nanoparticles using an herbal formulation, and to finalize the anti-inflammatory activity of silver nanoparticles. Result: Anti-inflammatory activity of silver nanoparticles show an incremental pattern with increase in concentration, where percentage of inhibition is high at 50μl. Its activity increases with increase in dosage. Conclusion: This study thus provides a strong base for understanding the anti-inflammatory activity of silver nanoparticles.
Keywords
Anti-inflammatory activity, Silver nanoparticles, Centella asiatica, Innovative technique, Eco friendly
Introduction
Centella asiatica, also known as Gotu Kola, Brahmi, Indian pennywort, and Asiatic pennywort, is a perennial herbaceous plant in the Apiaceae family of flowering plants. It is endemic to Asia's wetlands. It's a culinary vegetable as well as a medicinal plant [1]. Centella asiatica is mainly found in India, Pakistan, Sri Lanka, Madagascar, South Africa and Eastern Europe [2]. The major bioactive constituents are triterpene saponins mainly asiaticoside, sapogenin, asiatic acid, made-cassoside, and madecassic acid [3]. It is believed to have beneficial effects in improving memory and treating mental fatigue, eczema and anxiety. In Ayurveda, Centella is effectively used in the treatment of inflammation, blood disorders, anaemia, asthma, bronchitis, fever, urinary discharge, and splenomegaly [4]. Centella asiatica compounds have been considered to have a wide range of medicinal properties and therapeutic activity for a long time. The most important are antioxidant, anti-inflammatory, antimicrobial and anticarcinogenic activity [5]. Centella asiatica (Gotu Kola) is used in Indian systems of medicine for enhancing memory and these Herbal medicines can be used as adaptogens, these plant derived drugs either reduce stress reactions in the alarm phase and provide a certain degree of safety against long-term stress [6].In dermatology Centella asiatica is used in treatment of small wounds, hypertrophic wounds as well as burns, psoriasis and scleroderma [7]. As for a cosmetic purpose, Centella asiatica is used as an active compound in skin care preparations because of its antioxidant, antiinflammatory, anti-cellulite and antiaging activity [8]. As a result, Centella asiatica extract is a valuable raw material for a wide range of cosmetic applications [9].
Nanomaterials represent, materials that are natural, incidental, or manufactured, containing particles in their free state or which exist as aggregates, in which 50% or more of the particles in number, size, distribution, or one or more external dimension is in the size range of 1–100 nm [10]. They offer various physicochemical properties such as ultra-small size, large surface area, etc. These properties make them liable to reach and interact with the cellular, subcellular and even molecular level of the human body and hence it helps to achieve maximum therapeutic efficacy with minimal side effects [11]. The silver nanoparticles are widely used for the detection and treatment of diseases and treatment of diseases, drug delivery, and other purposes because they are eco-friendly [12].
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the most used drugs to treat pain and inflammatory conditions [13]. They do, however, have frequent side effects such as ulcers, bleeding, and renal problems. Therefore, medicinal plants are the common source of therapeutically active chemical substances with lesser side effects [14]. Our team has extensive knowledge and research experience that has translate into high quality publications [15-34]. The aim of the study is to evaluate the anti-inflammatory activity of Centella mediated silver nanoparticles
Materials and Methods
Preparation of Plant Extract
The Centella asiatica fruit is purchased and it is cleaned, dried and powdered into fine granules. 0.5 g of Centella asiatica powder are weighed separately and taken. Now 50 ml of distilled water is added to dissolve the weighted extract in conical flasks mixed well. This mixture is boiled at 60 degree Celsius for 7 minutes with the help of a heat mantle. Then the boiled extract is filtered with the help of filter paper. The filtered extract was collected and stored in 4C for further use (Figures 1 and 2).

Figure 1. Crude extract.
Figure 2. Boiling of extract.
Synthesis of nanoparticles
This Centella asiatica extract is treated with 0.016 g of silver nitrate and 90 ml of distilled water and it is placed in a semi-automatic shaker at 900 rpm. With the help of a double beam U-V spectrophotometer, the synthesis of nanoparticles for every one hour is noted (Figure 3).

Figure 3. Synthesized nanoparticles.
Characterization of silver nanoparticles
The synthesis of nanoparticles is primarily characterized using UV-vis spectroscopy. 3 ml of the solution is taken in a cuvette and scanned in a UV-vis spectrometer under 350 nm to 550 nm wavelength. The results were recorded for graphical analysis.
Preparation of silver nanoparticle powder
The Ag-NP solution was centrifuged using lark refrigerated centrifuges. The centrifugation was done at 8000 rpm for 10 min, and the pellet was collected and washed with distilled water twice. The final purified pellet is collected and dried at 60 C. Finally, the nanoparticles powder was collected and stored in an airtight Eppendorf tube.
Evaluation of anti-inflammatory activity using albumin denaturation assay:
2ml of 1% Bovine albumin fraction was mixed with 400μL of Centella asiatica mediated silver nanoparticles in different concentrations (10-50 μg/ml) and the PH of reaction mixture was adjusted to 6.8 using 1N HCL. The reaction mixture was incubated at room temperature for 20 minutes in a water bath. The mixture was cooled to room temperature and the absorbance value was recorded at 660nm. An equal amount of plant extract was replaced with DMSO for control. Diclofenac sodium in different concentrations was used as standard. The experiment was performed in triplicate (Figures 4 and 5).
Figure 4. Anti-inflammatory activity of Centella asiatica.
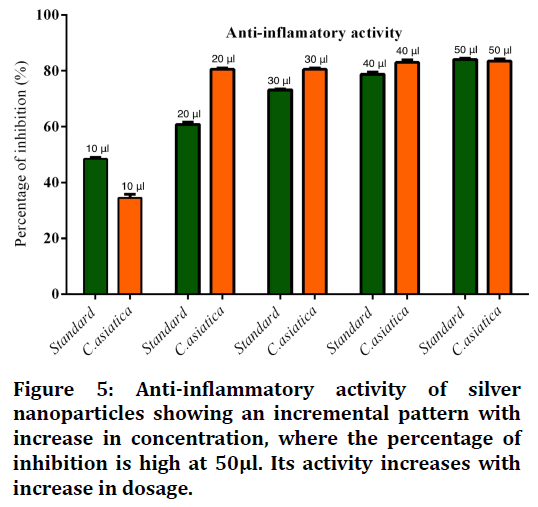
Figure 5. Anti-inflammatory activity of silver nanoparticles showing an incremental pattern with increase in concentration, where the percentage of inhibition is high at 50μl. Its activity increases with increase in dosage.
% Inhibition was calculated using the following formula
% Inhibition=Control O.D–Sample O.D/Control O.D
Were,
Control O.D=Optical density of control
Sample O.D=Optical density of test sample.
Results
Figure 5 shows anti-inflammatory activity of silver nanoparticles showing an incremental pattern with increase in concentration, where the percentage of inhibition is high at 50μl. Its activity increases with increase in dosage.
Discussion
A study showed that antiallergic activities have been obtained with all the extracts of C. asiatica comparable with that of standard drug ketotifen viz., 75% for sheep serum model and 69% for the compound 48/80 model. Glycosides which are normally present in the polar fraction have been reported to possess antiallergic potential [15]. Saha et al in their study showed Methanol extract of C. asiatica had significant anti-inflammatory effect against carrageenan-induced paw edema model, whereas chloroform extract had no such effect on rats. Carrageenan induced inflammation is a well-established method to detect orally active anti-inflammatory agents which shows biphasic response [35]. Bylka et al in their study showed that promoting fibroblast proliferation and increasing the synthesis of collagen as well as acidic mucopolysaccharides, increasing intra- cellular fibronectin content and mitotic activity in the germ layer, significantly improving the tensile strength of newly formed skin as well as by inhibiting the inflammatory phase of hypertrophic scars and keloids. His studies also suggest that the use of C. asiatica or its components may be useful in the treatment of psoriasis and scleroderma [9]. Hussin et al studies show the effect of C. asiatica juice on human HepG2 cell line using MTT assay, and it showed cytotoxic effects on tumor cells in a dosedependent manner. At a concentration above 0.1% of juice, a higher amount of DNA damage and apoptotic cell death was observed on human HepG2 cell lines [36]. Oyedeji OA et al in their study he observed that essential oil extract showed antibacterial properties against Grampositive (Bacillus subtilis and S. aureus) and Gramnegative (Escherichia coli, Pseudomonas aeruginosa, and Shigella sonnei) with MIC values ranging from 1.25 to 0.039 mg/ml [37]. Silver nanoparticles have an antibacterial effect due to its activity of forming pits in the cell wall of the bacteria. There is an evident increase in the cell permeability that will result in cell death [38]. Due to their potential applications in the medical sector, especially in the development of biodegradable surgical sutures, silver nanoparticles (Ag NPs) have a lot of attention [39]. Silver particles have various reactions with a bacterial cell, that are interaction with cell walls causing lysis, preventing DNA replication and also disrupts bacterial protein synthesis [40].
Conclusion
This research provokes the inflammatory action of silver nanoparticles produced from the plant extracts Centella. It is shown that it has a significant inhibitory activity with the least side effects. However, still, further research is required in knowing the benefits of these plants and also the mechanism behind their action in further studies. As a result, this analysis provides a strong basis for understanding silver nanoparticles' anti-inflammatory properties.
Acknowledgement
We thank the Saveetha Dental College for giving us the opportunity and support to conduct this study.
Source of Funding
The present study was supported by the following agencies.
Saveetha Dental College, SIMATS, saveetha university.
Royal hospital-Thanjavur.
References
- James JT, Dubery IA. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 2009; 14:3922-41.
- Brinkhaus B, Lindner M, Schuppan D, et al. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella aslatica. Phytomed 2000; 7:427-48.
- Cheng CL, Koo MW. Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci 2000; 67:2647-53.
- Hamid AA, Shah ZM, Muse R, et al. Characterisation of antioxidative activities of various extracts of Centella asiatica (L) Urban. Food Chem 2002; 77:465-9.
- Francis T, Rajeshkumar S, Roy A, et al. Anti-inflammatory and cytotoxic effect of arrow root mediated selenium nanoparticles. Pharmacog J 2020; 12.
- Wagner H, Nörr H, Winterhoff H. Plant adaptogens. Phytomed 1994; 1:63–76.
- Bylka W, Znajdek-Awizen P, Studzinska-Sroka E, et al. Centella asiatica in dermatology: An overview. Phytotherapy Res 2014; 28:1117-24.
- Mohapatra S, Leelavathi L, Rajeshkumar S, et al. Assessment of cytotoxicity, anti-inflammatory and antioxidant activity of zinc oxide nanoparticles synthesized using clove and cinnamon formulation--an in-vitro study. J Evolution Med Dent Sci 2020; 9:1859-65.
- Bylka W, Znajdek-Awizen P, Studzinska-Sroka E, et al. Centella asiatica in cosmetology. Adv Dermatol Allergology 2013; 30:46.
- Boopathi S, Gopinath S, Boopathi T, et al. Characterization and antimicrobial properties of silver and silver oxide nanoparticles synthesized by cell-free extract of a mangrove-associated Pseudomonas aeruginosa M6 using two different thermal treatments. Industrial Eng Chem Res 2012; 51:5976-85.
- Rajeshkumar S. Phytochemical constituents of fucoidan (Padina tetrastromatica) and its assisted AgNPs for enhanced antibacterial activity. IET Nanobiotechnol 2017; 11:292-9.
- Nasim I, Kumar SR, Vishnupriya V, et al. Cytotoxicity and anti-microbial analysis of silver and graphene oxide bio nanoparticles. Bioinformation 2020; 16:831–6.
- Conforti F, Sosa S, Marrelli M, et al. The protective ability of mediterranean dietary plants against the oxidative damage: The role of radical oxygen species in inflammation and the polyphenol, flavonoid and sterol contents. Food Chem 2009; 112:587–94.
- Gupta M, Mazumder UK, Gomathi P, et al. Antiinflammatory evaluation of leaves of Plumeria acuminata. BMC Complement Alternative Med 2006; 6:1-6.
- Princeton B, Santhakumar P, Prathap L. Awareness on preventive measures taken by health care professionals attending COVID-19 patients among dental students. Eur J Dent 2020; 14:S105–9.
- Mathew MG, Samuel SR, Soni AJ, et al. Evaluation of adhesion of Streptococcus mutans, plaque accumulation on zirconia and stainless steel crowns, and surrounding gingival inflammation in primary molars: randomized controlled trial. Clin Oral Investig 2020; 24:3275–80.
- Sridharan G, Ramani P, Patankar S, et al. Evaluation of salivary metabolomics in oral leukoplakia and oral squamous cell carcinoma. J Oral Pathol Med 2019; 48:299–306.
- Hannah R, Ramani P, Ramanathan A, et al. CYP2 C9 polymorphism among patients with oral squamous cell carcinoma and its role in altering the metabolism of benzo [a] pyrene. Oral Surg Oral Med Oral Pathol Oral Radiol 2020; 130:306-12.
- Antony JVM, Ramani P, Ramasubramanian A, et al. Particle size penetration rate and effects of smoke and smokeless tobacco products-An invitro analysis. Heliyon 2021; 7:e06455.
- Sarode SC, Gondivkar S, Sarode GS, et al. Hybrid oral potentially malignant disorder: A neglected fact in oral submucous fibrosis. Oral Oncol 2021; 105390.
- Ramani P, Tilakaratne WM, Sukumaran G, et al. Critical appraisal of different triggering pathways for the pathobiology of pemphigus vulgaris-A review. Oral Dis 2021.
- Chandrasekar R, Chandrasekhar S, Sundari KKS, et al. Development and validation of a formula for objective assessment of cervical vertebral bone age. Prog Orthod 2020; 21:38.
- Subramanyam D, Gurunathan D, Gaayathri R, et al. Comparative evaluation of salivary malondialdehyde levels as a marker of lipid peroxidation in early childhood caries. Eur J Dent 2018; 12:67–70.
- Jeevanandan G, Thomas E. Volumetric analysis of hand, reciprocating and rotary instrumentation techniques in primary molars using spiral computed tomography: An in vitro comparative study. Eur J Dent 2018; 12:21–6.
- Ponnulakshmi R, Shyamaladevi B, Vijayalakshmi P, et al. In silico and in vivo analysis to identify the antidiabetic activity of beta sitosterol in adipose tissue of high fat diet and sucrose induced type-2 diabetic experimental rats. Toxicol Mech Methods 2019; 29:276–90.
- Sundaram R, Nandhakumar E, Haseena Banu H. Hesperidin, a citrus flavonoid ameliorates hyperglycemia by regulating key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Toxicol Mech Methods 2019; 29:644–53.
- Alsawalha M, Rao CV, Al-Subaie AM, et al. Novel mathematical modelling of Saudi Arabian natural diatomite clay. Mater Res Express 2019; 6:105531.
- Yu J, Li M, Zhan D, et al. Inhibitory effects of triterpenoid betulin on inflammatory mediators inducible nitric oxide synthase, cyclooxygenase-2, tumor necrosis factor-alpha, interleukin-6, and proliferating cell nuclear antigen in 1, 2-dimethylhydrazine-induced rat colon carcinogenesis. Pharmacogn Mag 2020; 16:836.
- Shree KH, Ramani P, Sherlin H, et al. Saliva as a diagnostic tool in oral squamous cell carcinoma–a systematic review with meta-analysis. Pathol Oncol Res 2019; 25:447-53.
- Zafar A, Sherlin HJ, Jayaraj G, et al. Diagnostic utility of touch imprint cytology for intraoperative assessment of surgical margins and sentinel lymph nodes in oral squamous cell carcinoma patients using four different cytological stains. Diagn Cytopathol 2020; 48:101–10.
- Karunagaran M, Murali P, Palaniappan V, et al. Expression and distribution pattern of podoplanin in oral submucous fibrosis with varying degrees of dysplasia–an immunohistochemical study. J Histotechnol 2019; 42:80-6.
- Sarode SC, Gondivkar S, Gadbail A, et al. Oral submucous fibrosis and heterogeneity in outcome measures: A critical viewpoint. Future Oncol 2021; 17:2123–6.
- Raj Preeth D, Saravanan S, Shairam M, et al. Bioactive Zinc(II) complex incorporated PCL/gelatin electrospun nanofiber enhanced bone tissue regeneration. Eur J Pharm Sci 2021; 160:105768.
- Prithiviraj N, Yang GE, Thangavelu L, et al. Anticancer compounds from starfish regenerating tissues and their antioxidant properties on human oral epidermoid carcinoma KB cells. Pancreas 2020; 49:155-156.
- Saha S, Guria T, Singha T, et al. Evaluation of analgesic and anti-inflammatory activity of chloroform and methanol extracts of Centella asiatica Linn. ISRN Pharmacol 2013; 2013:789613.
- Hussin F, Eshkoor SA, Rahmat A, et al. The centella asiatica juice effects on DNA damage, apoptosis and gene expression in hepatocellular carcinoma (HCC). BMC Complement Altern Med 2014; 14:32.
- Oyedeji OA, Afolayan AJ. Chemical composition and antibacterial activity of the essential oil of Centella asiatica. Growing in South Africa. Pharm Biol 2005; 43:249-52.
- Abd-Elsalam KA, Prasad R. Nanobiotechnology applications in plant protection. Springer 2018; 394.
- Kohsari I, Shariatinia Z, Pourmortazavi SM. Antibacterial electrospun chitosan-polyethylene oxide nanocomposite mats containing bioactive silver nanoparticles. Carbohydr Polym 2016; 140:287–98.
- Shathviha PC, Ezhilarasan D, Rajeshkumar S, et al. ß-sitosterol Mediated silver nanoparticles induce cytotoxicity in human colon cancer HT-29 Cells. Avicenna J Med Biotechnol 2021;13:42–6.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
S Ganesh1, Abirami Arthanari1* and S Rajeshkumar2
1Department of Forensic Odontology, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India2Department of Pharmacology, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India
Received: 27-Dec-2021, Manuscript No. JRMDS-21-41700; , Pre QC No. JRMDS-21-41700 (PQ); Editor assigned: 29-Dec-2022, Pre QC No. JRMDS-21-41700 (PQ); Reviewed: 12-Jan-2022, QC No. JRMDS-21-41700; Revised: 17-Jan-2022, Manuscript No. JRMDS-21-41700 (R); Published: 24-Jan-2022
