Research - (2022) Volume 10, Issue 4
Antimicrobial Efficacy of Dual Wavelength Lasers-Activated Irrigation in Root Canal Therapy (In vitro Study)
Hani Nassar, Leena Merdad, Mohammed Zahran and Khalid Merdad*
*Correspondence: Khalid Merdad, Department of Endodontics, King Abdulaziz University, Jeddah, KSA, Email:
Abstract
This study evaluated the antimicrobial efficacy of Erbium chromium and diode lasers, with or without irrigant, against Enterococcus faecalis (E. faecalis) biofilm. Sixty-five single rooted teeth were incubated with E. faecalis and then treated either with: 5.25% sodium hypochlorite (Group 1); 5.25% sodium hypochlorite then 2% chlorhexidine (Group 2); dual laser with saline (Group 3); dual laser with 5.25% sodium hypochlorite (Group 4); or dual laser with 5.25% sodium hypochlorite then 2% chlorhexidine (Group 5). Positive control teeth were incubated with E. faecalis but received no antimicrobial treatment while negative control teeth were left uninfected. Decontamination was assessed by estimation of colony forming units before and after irrigation using a manual colony counter and Open CFU software. Treatments 1, 2, 4 and 5 achieved 92 to 100% decontamination with no significant difference between these treatments (P>0.005). Dual laser with saline resulted in significantly less disinfection (75%) than the other treated groups (P<0.005).Keywords
Diode laser, Enterococcus faecalis, Er, Cr: YSGG, laser activated irrigation, Root canal disinfection
Introduction
Root canal treatment is a multistep procedure aiming to avert or treat existing apical periodontist. According to Kakehashi et al. [1], microorganisms are the prime causative factor for apical periodontitis. Hence, bacterial elimination during endodontic therapy can significantly reduce periradicular infection [2]. Instrumentation techniques alone fail to promote complete canal decontamination, leaving 35% of the canal wall untouched [3]. Adjunctive chemical irrigation and/or medication alongside mechanical instrumentation is therefore of prime importance. Sodium hypochlorite (NaOCl) is a commonly utilized cost effective irrigation solution with superior tissue dissolving capability and strong antibacterial effect against microorganisms in planktonic and sessile forms [4]. However, its action is limited toward microorganisms residing deep inside dentinal tubules, thus possibly cause treatment failure [5]. Adjunctive methods have been advocated to improve the efficacy of irrigants, including hand agitation, sonic activation, ultrasonication, negative-pressure systems such as EndoVac and lasers [6]. Laser treatment has been proposed as an alternative/additive to chemical irrigation procedures during endodontic therapy [7]. It has the ability to clean and disinfect the root canal system against the most resistance microorganism, Enterococcus faecalis (E. faecalis) [8-10]. Laser activated irrigation (LAI) is based on actively agitating the irrigation solution with transferring pulse energy. These impulses, in the form of photo thermal and photomechanical waves, can disturb the microbial biofilm and target the bacterial cell wall, causing cellular swelling and rupture [11].
In endodontics, the most commonly employed laser wavelengths are diode (810 nm, 940 and 980 nm) and erbium family; including 2,780 nm yttrium- scandium-gallium-garnet (Er,Cr:YSGG) and erbium-doped yttrium aluminum garnet (Er:YAG) at 2,940 nm. There is significant evidence supporting the antimicrobial effect and smear layer removal of laser assisted endodontic disinfection. Diode lasers have affinity for melanin and pigmentation, targeting bacteria residing within the root canal space. Erbium lasers, especially 2,780 nm Er,Cr:YSGG, have demonstrated strong affinity to water and hydroxyapatite causing surface ablation of dentin and removal of the smear layer in addition to its antimicrobial properties [12].
The antimicrobial effect of single wavelength laser treatment, as a substitute or additive to contemporary endodontic disinfection procedures, has been demonstrated. Afkhami et al. [13] reported 97% microbial reduction of high-power diode laser irradiation when used alone against E. faecalis. However, recently there has been a shift toward the use of dual wavelength lasers (2,780 nm Er,Cr:YSGG and 940 nm diode) in several endodontic research disciplines [14,15]. Dual laser irradiation was found to achieve superior dentin permeability, hence, superior antimicrobial disinfection [14]. Moreover, Gutknecht et al. [15] investigated the antimicrobial effect of dual wavelength lasers against E. faecalis inoculated bovine dentin slices in vitro. They reported a 3.2 logarithmic reduction in infection when using dual wavelength lasers at 1,000 μm thickness of dentin. Still, the efficacy of dual laser procedures for the disinfection of root canals is not clear. Thus, the aim of the present study was to evaluate the antimicrobial efficacy of combined 2,780 nm Er,Cr:YSGG and 940 nm diode laser activated irradiation against E. faecalis biofilm with or without commonly utilized endodontic disinfecting solutions.
Materials and Methods
Specimen preparation
Experimental procedures were approved by the King Abdulaziz University (REC-FD #034-03-17) ethical committee. Experimental procedure took around 8 weeks from January 2020 to March 2020. Sixty-five extracted human single-rooted teeth with curvatures ≤ 15° were used. Crowns were decoronated at the cement enamel junction. Then canals were instrumented to full working length using Reciproc R40 (40/0.06) (VDW, Munich, Germany). During instrumentation, canals were irrigated with 5.25% NaOCl and smear layer was removed by treatment with 17% EDTA for 1 minute. Teeth apices were sealed with light-cured composite resin (Filtek™ Supreme, MN, USA) and the external root surfaces were coated with nail polish except for the cervical opening. Each specimen was placed in a 1 ml cryo-tube held in place with polyvinyl impression material (Ivoclar Vivadent Inc., NY, USA) and autoclaved at 134°C for 30 minutes.
Bacterial inoculation
Fresh E. faecalis (ATCC 19443) culture was prepared in sheep blood agar by incubation overnight at 37 ℃ until a McFarland turbidity of 0.5-0.6 determined by DensiCHEK™ PLUS (bioMérieux SA, France) was reached. Instrumented root canals, except for the negative control teeth (n=5), were inoculated with E. faecalis along with thioglycolate-hemin broth media (Saudi prepared media laboratory company, Ltd., KSA), for 4 weeks with media being refreshed every 72 hours. Five specimens were then longitudinally sectioned under sterile condition and prepared for Scanning Electron Microscopy (SEM) analysis to confirm biofilm growth.
Laser preparation
Two laser systems were utilized for disinfection procedures; Er,Cr:YSGG (Waterlase iPlus, Biolase, USA) laser emitting at 2,780 nm and diode laser system (iLase, Biolase, USA) emitting at 940 nm wavelength. The power settings for the Er,Cr:YSGG were Pave=0.75 W for radial firing tip (RFT)2 with diameter 200 μm/21 mm length and Pave=1.5 W for RFT3 with diameter 320 μm/17mm length. The frequency was fixed at 20 Hz and the pulse duration was 60 μs, with 10% water, while the air was off. For the diode laser, Pave=1.5 W, at continuous mode and energy (E) 2 tip with a diameter of 200 μm/20 mm length was used. The power settings were subjected to calibration factors of each fiber tip (RFT2 and E2=0.55 and RFT3=0.85), giving actual power settings for Er,Cr:YSGG of 0.41 W for RFT2 and 1.28 W for RFT3, and 0.83 W for the diode laser. One cycle of Er,Cr:YSGG was applied with tips placed 1 mm from the working length and withdrawn in an apico-coronal spiral motion at a rate of 2 mm/sec followed by 2 cycles of 940 nm diode laser in the same pattern resulting in a total laser irradiation time of 24 seconds. The choice of three laser cycles with a total of 24 seconds was based on a pilot study which showed a rapid decay of the RFT after a single usage of Er,Cr:YSGG laser beam emission and two times after diode laser emission.
Test groups
Teeth samples were randomly allocated to the treatment groups and control. Negative control teeth (n=5) received canal enlargement, using the same method as for experimental tooth samples, but were not infected. For the disinfection procedures, specimens were divided into five experimental groups (n=10) and one positive control (n=5) group, which received no disinfection treatment. The root canals were disinfected as follows: Group 1, syringe irrigation with 2.5 ml 5.25% NaOCl followed by 17% EDTA and final flush with 2.5 ml 5.25% NaOCl with a total of 2 minutes irrigation; Group 2, syringe irrigation with 5ml of 5.25% NaOCl, followed by 2 ml of saline irrigation and a final flush with 2.5 ml of 2% chlorhexidine for 2 minutes; Group 3, dual laser was applied in the presence of a few drops of saline; Group 4 dual laser was applied in the presence of a few drops of 5.25% NaOCl; Group 5, root canals were treated with dual laser in the presence of 5.25% NaOCl, then flushed with saline and reactivated in the presence of 2% CHX for a total of 24 seconds activation time.
Microbiological analysis
Bacterial samples were collected before and after disinfection procedures according to the methodology of Afkhami et al. [13]. Excess broth was first aspirated from the canal space using a sterile needle, and 0.1 ml of sterile saline applied with a new sterile needle. The dentin wall was scraped using a sterile K-file size 40, followed by insertion of sterile paper point size 40 for 30 seconds. The paper point was then transferred to a sterile test tube filled with 1 ml of saline and vortexed (VX-200, Labnet international, USA) for 20 seconds. A 10-fold serial dilution was prepared up to 10-4. Lastly, 150 μl of the 10-2 dilutions was spiral plated into sheep blood agar and incubated at 35° C for 24 hours. Each culture plate was counted manually using colony counter and transformed into actual counts based on the dilution factor. Finally, high resolution photographs from each plate were run through Open CFU software for automated counting.
Statistical analysis
Data were analyzed using SPSS version 22.0 (IBM, USA). Correlation between automated Open CFU software counts and manual colony counter device counts was tested using Spearman’s rho test. After confirming good correlation between them, manual colony counter device counts were used for further analyses. Median CFU counts before and after treatment was determined and percentage of microbial reduction (%MR) was calculated where:
% MR=(CFU (before treatment)-CFU (after treatment))/ (CFU (before treatment) X 100
Significant differences in percentage of microbial reduction between groups were identified using the Kruskal-Wallis test with post hoc Bonferroni test, considering a P value of <0.005 to be significant, correcting for pair-wise comparison.
Results
SEM biofilm confirmation & correlation analysis
After 4 weeks of E. faecalis incubation, SEM evaluation confirmed biofilm development seen as dense aggregates of bacteria biofilm attached to the internal wall of the root surface (Figure 1). Spearman’s rho test showed a significant correlation between colony counts with a manual colony counter device and Open CFU software {r=0.972 (P<0.01)}. Final data were analysed using the manual colony counter device.
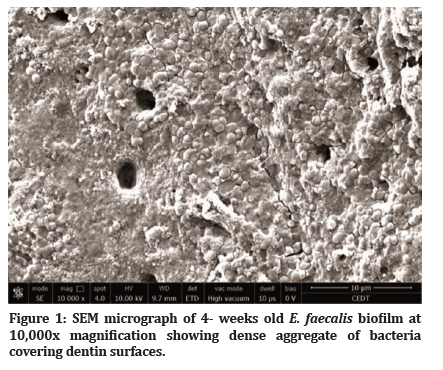
Figure 1: SEM micrograph of 4- weeks old E. faecalis biofilm at 10,000x magnification showing dense aggregate of bacteria covering dentin surfaces.
Microbial count before and after treatment
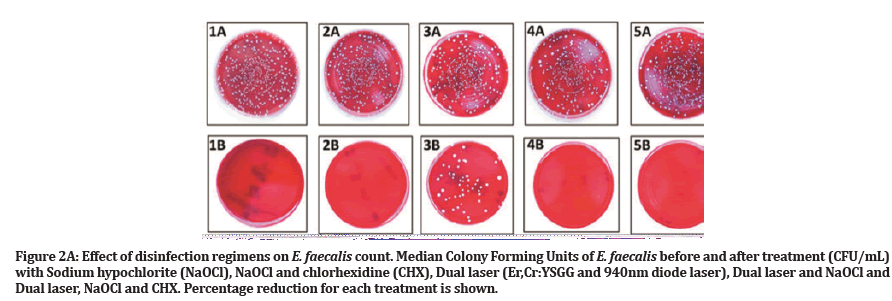
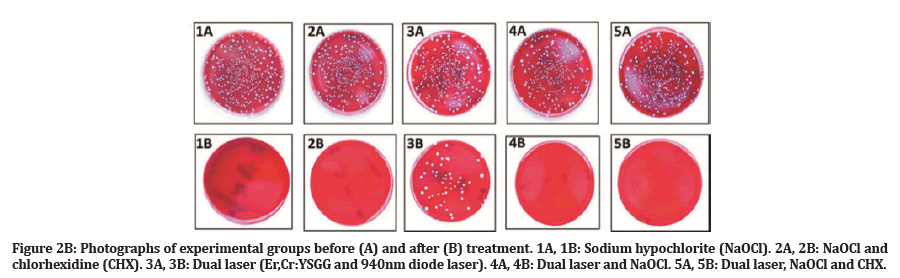
Negative control tooth samples showed no microbial growth. During the experiment, one sample was lost due to technical error in group 2, so the total number of specimens was adjusted to n=49. Descriptive statistics for the CFU count before and after treatment and percentage of reduction are presented in Table 1. The Kruskal Wallis test indicated a significance difference in the percentage of E. faecalis reduction across all treatment groups (P<0.001). After treatment, median percentages of E. faecalis elimination were as follows: 100% for group 1, group 2 and group 5; 92 % for group 4; and 75% for group 3. There were no significant differences between groups 1, 2, 4 and 5 (P>0.005). However, a significant difference was found between the aforementioned groups and group 3 (P<0.005) (Figure 2A and 2B).
| Treatment Groups | Before Treatment | After Treatment | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Reduction (%) | |
| NaOCl (n=10) | 2.78 x104 (6.8 X 104) | 0 | 100.00a |
| NaOCl+CHX (n=9) | 2.75 x104 (3.61 X 104) | 0 | 100.00a |
| Dual Laser+Saline (n=10) | 2.89 x104 (5.75 X 104) | 61.00 X 102 (189 X 104) | 75.00b |
| Dual Laser+NaOCl (n=10) | 2.55 x104 (5.46 X 104) | 0.5 X 102 (55 X 102) | 100.00a |
| Dual Laser+NaOCl+CHX (n=10) | 3.03 x104 (6.09 X 104) | 0 | 100.00a |
| Bacterial colony counts before and after indicated treatments. Percentage reduction in count is shown | |||
| †CFU/ml, colony forming unit per millilitre; NaOCl, Sodium hypochlorite; CHX, chlorhexidine; IQR: Interquartile range | |||
| ‡Different letters indicate statistically significant difference (p<0.005, Post- hoc test) | |||
Table 1: Effect of disinfection regimens on E. faecalis count.
Figure 2A:Effect of disinfection regimens on E. faecalis count. Median Colony Forming Units of E. faecalis before and after treatment (CFU/mL) with Sodium hypochlorite (NaOCl), NaOCl and chlorhexidine (CHX), Dual laser (Er,Cr:YSGG and 940nm diode laser), Dual laser and NaOCl and Dual laser, NaOCl and CHX. Percentage reduction for each treatment is shown.
Figure 2B:Photographs of experimental groups before (A) and after (B) treatment. 1A, 1B: Sodium hypochlorite (NaOCl). 2A, 2B: NaOCl and chlorhexidine (CHX). 3A, 3B: Dual laser (Er,Cr:YSGG and 940nm diode laser). 4A, 4B: Dual laser and NaOCl. 5A, 5B: Dual laser, NaOCl and CHX.
Discussion
Elimination of intracanal bacteria always remains the major goal of root canal therapy. Bacterial presence is a challenge, and its elimination is crucial in treating existing, or preventing post treatment, apical periodontitis. Despite all advances in the field, the search continues for the ideal root canal disinfectant that produces three-dimensional canal sterility. Laser disinfection is of interest, with the bactericidal effect of single laser treatment, with or without chemical solutions, being demonstrated [16].
To our knowledge, the present study is the second attempt to evaluate the antimicrobial effect of dual lasers (2,780 nm Er,Cr:YSGG and 940 nm diode). In a study by Gutknecht et al. [15], dentin slices of variable thicknesses were inoculated with E. faecalis. In the present study, extracted human teeth were utilized in order to simulate the clinical setting [13].
E. faecalis was chosen because of its extensive use for antimicrobial studies [12]. E. faecalis is a gram positive facilitative anaerobic species with multiple survival and resistance mechanisms against intracanal medications [17]. Moreover, the presence of E. faecalis has been associated with failure in 67-89.6% of patients with secondary endodontic treatment [17]. It travels deep into the dentinal tubules (up to 250μm) making disinfection a challenging procedure [18]. One of its important survival mechanisms is the ability to form biofilm [19].
The time needed for E. faecalis to colonize biofilm inside the root canal was inconstant across the studies. Some studies used only a few hours incubation time [20], while others used a few days [21]. In this study, 4-weeks incubation time was used as recommended by Saber and El-Hady [22] resulting in consistent colonization as confirmed by SEM micrograph (Fig 1). Moreover, Du et al. [23] and Wang et al. [24] found that 3-weeks-old E. faecalis biofilm resisted the chemical action of the disinfection solutions.
It has been suggested that the combination of 2,780 nm Er,Cr:YSGG and 940 nm diode laser as a laser assisted endodontic disinfection protocol yields superior microbial elimination owing to the different absorption coefficients of these two wavelengths for the target tissues. According to the Beer-Lambert law, the Er,Cr:YSGG laser has a high absorption coefficient [25] that provides smear layer disruption [16]. This then facilitates low absorption coefficient diode laser penetration, more than 500 μm into dentin, decontaminating deep residing bacteria [8].
Together, 2,780 nm Er,Cr:YSGG and 940 nm diode laser exhibited a substantial antimicrobial effect, with or without chemical disinfectants [16,21]. Gutknecht et al. [15] reported that dual wavelength laser (2,780 nm Er,Cr:YSGG and 940 nm diode) exhibited a significant 3.2 logarithmic reduction of E. faecalis (~99.9%) in 1,000 μm dentin thickness, compared to Er,Cr:YSGG irradiation alone, which achieved an average of 2.1 logarithmic reduction (99.2%). This may be attributed to the direct laser irradiation technique causing microbial destruction via thermolysis. In the current study, LAI technique was used in combination with commonly utilized endodontic irrigants. As discussed above, the dual laser technique may be advantageous due to optimal disruption of the biofilm, due to photo thermal and photomechanical processes in which acoustic streaming phenomena cause thermo mechanical disruption of the microbial biofilm, facilitating penetration of the diode laser.
This study found that maximum bacterial reduction was obtained by 5.25% NaOCl alone or in combination with CHX, and that dual wavelength laser treatment did not enhance their action. All treatment regimens, except dual laser therapy alone, were able to completely eradicate bacteria with no significant difference between them (P>0.005). These results are in line with previous studies [9,10]. In contrast, Peters et al. [26] have shown that low powered LAI (Er:YAG) with 6% NaOCl generated significantly more negative samples than 6% NaOCl syringe irrigation or ultrasonic activation. Similarly, qualitative SEM analysis showed that Er,Cr:YSGG laser energized 4% NaOCl had greater E. faecalis biofilm elimination in the canal lumen and inside dentinal tubules than syringe and sonic activation [27]. The differences between these studies and the current study may be attributed to microbial inoculation methodology, sampling technique; laser wavelength used or laser parameters including power, number of cycles and total irradiation time. This recognition is important as taken together these findings emphasize the importance of optimizing these laser parameters in order to achieve optimal decontamination.
Although NaOCl alone showed complete canal decontamination, this should be interpreted with caution as it is proven that dentin components are able to buffer its antimicrobial effect in the clinical setting [28]. Moreover, although the present findings show that the addition of dual laser activation to the use of NaOCl and CHX alone did not increase microbial decontamination, dual lasers have been shown to achieve canal decontamination in a shorter time frame, volume and greater penetration depth than chemical disinfectants alone [8].
Canal morphology is another important parameter. The large apical instrumentation and limited canal curvature used in this study possibly facilitated chemical decontamination, resulting in substantial efficacy for chemical reagents that laser treatment was unlikely to improve.
The difference in the volume of irrigant used between the groups receiving chemical treatment alone and chemical treatment augmented with laser therapy is acknowledged. A lower volume of chemical irrigant was used with dual laser activation (1ml versus 5 ml NaOCl and 0.5 ml versus 2.5 ml CHX), yet equivalent microbial decontamination was seen. This may be attributed to the hydrodynamic phenomenon formed by the acoustic streaming and thermolysis of laser mechanics [11].
The dual laser energized saline exhibited significantly lower decontamination (75%) in comparison to other groups. The use of saline in this study was intended to examine the physical effect of dual LAI as a possible substitute for chemical disinfection. Seet et al. [27] demonstrated that 60 second Er,Cr:YSGG energized saline treatment to cause a marked disruption of mature E. faecalis biofilm. Nonetheless, they found dense aggregates of E. faecalis present inside the dentinal tubules indicating the limited usefulness of saline laser energization. Our findings are consistent with them.
Technical considerations may be essential in achieving improved decontamination with laser therapy. RFT tips (200 and 320 μm) used in the present study have a calibration factor of 0.55 and 0.85 respectively. This would mean that for 0.75 W and 1.5 W used for Er, Cr:YSGG and 1.5 W for diode laser, the output power attenuated to 0.41 W and 1.28 W for Er,Cr:YSGG and 0.825 W for diode laser. This could explain the limited usefulness of dual laser treatment. Thus, improved decontamination can only be seen when all these parameters are optimized. Furthermore, the cell wall structure of E. faecalis may be particularly resistant to the heat generated by laser irradiation [29].
Data regarding the outcome of laser-assisted endodontic treatment is yet controversial. Martins et al. [30] demonstrated no significant difference in the healing outcome of periapical lesion 6 months after conventional root canal therapy using 3% NaOCl and Er,Cr:YSGG assisted endodontic therapy. However, in 2018 the same group reported successful outcomes treating necrotic teeth endodontically using low powered dual wavelength laser (2,780 nm Er,Cr:YSGG (0.69 W) and 940 nm diode lasers (0.55 W) with only sterile saline and no chemical irrigants or intracanal dressing. They showed an immediate remission of pain and radiographic signs of increased bone density after 12 months follow-up [12].
The endodontic literature is still lacking well-constructed clinical trials with long term treatment outcomes to definitively support laser assisted endodontic therapy. Laboratory studies on single and dual wavelength laser activation have been encouraging. However, this study questions the effectiveness of dual wavelength laser activation as adjunctive therapy in endodontic procedures. Well-controlled, carefully designed, randomized controlled clinical trials are needed to definitively address the benefits of the use of laser therapy in endodontic therapy.
Conclusion
Laser assisted endodontic therapy with dual wavelength laser (2,780 nm Er,Cr:YSGG and 940 nm diode) is of interest as an additive to contemporary endodontic irrigants. In this study a comparable antimicrobial efficacy was noted between NaOCl, NaOCl/CHX with or without dual laser activation. Dual wavelength laser activation used without chemical solutions showed inferior antimicrobial effect. Given the conflicting reports in the literature and the potential for clinical parameters to affect the outcomes, further in-vivo studies are needed to generate definitive clinically relevant results.
Acknowledgement
The authors acknowledge with thanks Deanship of Scientific Research (DSR) for their technical and financial support. We would like to thank Dr. Alaa Sultan for his help and supervision during Erbium chromium yttriumscandium- gallium-garnet (Er,Cr:YSGG) and diode laser manipulations.
Authors Contributions
Conceptualization: Reham Baothman, Sawsan Abu Zeid, Khalid Merdad
Data curation: Reham Baothman, Sawsan Abu Zeid
Formal analysis: Reham Baothman, Sawsan Abu Zeid, Hani Nassar, Leena Merdad and Mohammed Zahran
Funding acquisition: Reham Baothman and Khalid Merdad
Investigation: Reham Baothman
Methodology: Reham Baothman
Project administration: Reham Baothman
Resources: Reham Baothman, Sawsan Abu Zeid and Khalid Merdad
Software: Reham Baothman, Sawsan Abu Zeid and Khalid Merdad
Supervision: Sawsan Abu Zeid, Khalid Merdad, Hani Nassar and Leena Merdad
Validation: Reham Baothman, Sawsan Abu Zeid and Mohammed Zahran
Visualization: Reham Baothman
Writing original draft: Reham Baothman
Writing review and editing: Reham Baothman, Sawsan Abu Zeid, Khalid Merdad and Mohammed Zahran
Conflicts of Interest
Authors declare that there is no conflict of interest.
Funding Statement
The study was funded by King Abdulaziz University (G-574-165-1437) and ethical approval was obtained from the King Abdulaziz University Faculty of Dentistry Research Ethical Committee (034-03-17).
Ethical Approval
Experimental procedures were approved by the King Abdulaziz University (REC-FD #034-03-17) ethical committee.
References
- Kakehashi S, Stanley H, Fitzgerald R. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg Oral Med Oral Pathol 1965; 20: 340-349.
- Sjögren U, Figdor D, Persson S, et al. Influence of infection at the time of root filling on the outcome of endodontic treatment of teeth with apical periodontitis. Int Endod J 1997; 30:297-306.
- Peters OA, Schonenberger K, Laib A. Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 2001; 34:221-230.
- Zehnder M. Root canal irrigants. J Endod 2006; 32:389-398.
- Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod 1997; 23:725-727.
- Gu LS, Kim JR, Ling J, et al. Review of contemporary irrigant agitation techniques and devices. J Endod 2009; 35:791-804.
- Gutknecht N. Lasers in endodontics. J Laser Health Acad 2008; 4:1-5.
- Gutknecht N, Franzen R, Schippers M, et al. Bactericidal effect of a 980-nm diode laser in the root canal wall dentin of bovine teeth. J Clin Laser Med Surg 2004; 22:9-13.
- Pedulla E, Genovese C, Campagna E, et al. Decontamination efficacy of photon‐initiated photoacoustic streaming (pips) of irrigants using low‐energy laser settings: An ex vivo study. Int Endod J 2012; 45:865-870.
- Cretella G, Lajolo C, Castagnola R, et al. The effect of diode laser on planktonic Enterococcus faecalis in infected root canals in an ex vivo model. Photomed Laser Surg 2017; 35:190-194.
- Blanken JW, Verdaasdonk RM. Cavitation as a working mechanism of the er,cr:Ysgg laser in endodontics: A visualization study. J Oral Laser Appl 2007; 7:97-106.
- Martins MR, Franzen R, Depraet F, et al. Rationale for using a double-wavelength (940 nm+ 2780 nm) laser in endodontics: Literature overview and proof-of-concept. Lasers Dent Sci 2018; 2:29-41.
- Afkhami F, Akbari S, Chiniforush N. Entrococcus faecalis elimination in root canals using silver nanoparticles, photodynamic therapy, diode laser, or laser-activated nanoparticles: An in vitro study. J Endod 2017; 43:279-282.
- Al-Karadaghi TS, Franzen R, Jawad HA, et al. Investigations of radicular dentin permeability and ultrastructural changes after irradiation with er, cr: Ysgg laser and dual wavelength (2780 and 940 nm) laser. Lasers Med Sci 2015; 30:2115-2121.
- Gutknecht N, Al-Karadaghi TS, Al-Maliky MA, et al. The bactericidal effect of 2780 and 940 nm laser irradiation on Enterococcus faecalis in bovine root dentin slices of different thicknesses. Photomed Laser Surg 2016; 34:11-16.
- Beer F, Buchmair A, Wernisch J, et al. Comparison of two diode lasers on bactericidity in root canals—an in vitro study. Lasers Med Sci 2012; 27:361-364.
- Rocas IN, Siqueira JF, Santos KR. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod 2004; 30:315-320.
- Schäfer E, Bössmann K. Antimicrobial efficacy of chlorhexidine and two calcium hydroxide formulations against Enterococcus faecalis. J Endod 2005; 31: 53-56.
- Distel JW, Hatton JF, Gillespie MJ. Biofilm formation in medicated root canals. J Endodont 2002; 28: 689-693.
- Meire M, Coenye T, Nelis H, et al. Evaluation of nd: Yag and er: Yag irradiation, antibacterial photodynamic therapy and sodium hypochlorite treatment on Enterococcus faecalis biofilms. Int Endod J 2012; 45:482-491.
- Eldeniz AU, Ozer F, Hadimli HH, et al. Bactericidal efficacy of er,cr:Ysgg laser irradiation against Enterococcus faecalis compared with naocl irrigation: An ex vivo pilot study. Int. Endod J 2007; 40:112-119.
- Saber SE-DM, El-Hady SA. Development of an intracanal mature Enterococcus faecalis biofilm and its susceptibility to some antimicrobial intracanal medications; an in vitro study. Eur J Dent 2012; 6:43.
- Du T, Wang Z, Shen Y, et al. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. J Endod 2014; 40:509-514.
- Wang Z, Shen Y, Haapasalo M. Effectiveness of endodontic disinfecting solutions against young and old Enterococcus faecalis biofilms in dentin canals. J Endod 2012; 38:1376-1379.
- Diaci J, Gaspirc B. Comparison of er: Yag and er, cr: Ysgg lasers used in dentistry. J Laser Health Acad 2012; 1:1-13.
- Peters OA, Bardsley S, Fong J, et al. Disinfection of root canals with photon-initiated photoacoustic streaming. J Endod 2011; 37: 1008-1012.
- Seet AN, Zilm PS, Gully NJ, et al. Qualitative comparison of sonic or laser energisation of 4% sodium hypochlorite on an Enterococcus faecalis biofilm grown in vitro. Aust Endod J 2012; 38:100-106.
- Haapasalo H, Siren E, Waltimo T, et al. Inactivation of local root canal medicaments by dentine: An in vitro study. Int Endod J 2000; 33:126-131.
- Schoop U, Kluger W, Moritz A, et al. Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med 2004; 35: 111-116.
- Martins MR, Carvalho MF, Vaz I, et al. Efficacy of er, cr: Ysgg laser with endodontical radial firing tips on the outcome of endodontic treatment: Blind randomized controlled clinical trial with six-month evaluation. Lasers Med Sci 2013; 28:1049-1055.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Hani Nassar, Leena Merdad, Mohammed Zahran and Khalid Merdad*
1Department of Endodontics, Prince Sultan Military Medical City, Riyadh, KSADepartment of Endodontics, King Abdulaziz University, Jeddah, KSA
2Department of Endodontics, Cairo University, Cairo, KSA
3Department of Restorative Dentistry, King Abdulaziz University, Jeddah, KSA
4Department of Dental public Health, King Abdulaziz University, Jeddah, KSA
5Department of Oral and Maxillofacial Rehabilitation, King Abdulaziz University, Jeddah, KSA
Citation: Reham Baothman, Sawsan Abu Zeid, Hani Nassar, Leena Merdad, Mohammed Zahran, Khalid Merdad, Antimicrobial Efficacy of Dual Wavelength Lasers-Activated Irrigation in Root Canal Therapy (In vitro study), J Res Med Dent Sci, 2022, 10 (4):121-126.
Received: 01-Apr-2022, Manuscript No. JRMDS-22-61080; , Pre QC No. JRMDS-22-61080 (PQ); Editor assigned: 04-Apr-2022, Pre QC No. JRMDS-22-61080 (PQ); Reviewed: 18-Apr-2022, QC No. JRMDS-22-61080; Revised: 22-Apr-2022, Manuscript No. JRMDS-22-61080 (R); Published: 29-Apr-2022