Research - (2022) Volume 10, Issue 1
Antioxidant Potential of Opuntia ficus Fruit Extracts
Ashok K1*, Babu M1, Kalaiarasi V2, Muthukumaravel A2 and Padmapriya G3
*Correspondence: Ashok K, Department of Microbiology and Biotechnology, Faculty of Arts and Science College, India, Email:
Abstract
The DPPH results indicate that methanol extract showed more antioxidant potential than the other solvent extract. Antioxidant activity of Opuntia ficus fruit extract found to be in the following order of Methanol extract (66.20 μg/ml) ˂chloroform extract (68.085 μg/ml) ˂ethyl acetate (75.468 μg/ml) ˂Aqueous extract (127.88 μg/ml) ˂Hexane (250.078 μg/ml). There were significance differences (P<0.05) among the extracts and the standard Ascorbic acid at different concentration (μg/ml) this may be due to the radical scavenging of the extracts and the antioxidant mechanism might have involved in it. The DPPH assay indicated that the methanol extract has the ability to scavenge free radicals and the capacity to decolorize DPPH the controls. This can, as well, be attributed to the presence of polyphenolic compounds.
Keywords
Opuntia ficus, DPPH, Two-way ANOVA, SPSS software
Introduction
Epidemiological studies show that increased consumption of fruit and vegetables is associated with a lower risk of degenerative diseases [1,2].
The current lifestyle leads to the production of free radicals and reactive oxygen species. Natural antioxidants protect against oxidative stress and related diseases therefore play an important role in health care [3].
The main source of natural antioxidants is plant foods. Fruits and vegetables are important sources of antioxidant polyphenols for humans [4]. Fruits are rich in polyphenols and antioxidant activity is expected to be high due to their low moisture content and longer shelf life. Recently, natural antioxidants have gained considerable interest among nutritionists, food producers and consumers because of their expected safety and potential therapeutic value [5]. Available data on the antioxidant activity and phenol content of fruits commonly consumed in India have so far been neglected as functional foods [6,7]. Against this background, the antioxidant potential of Opuntia ficus fruit extracts was analysed.
Materials and Methods
Collection of identification of plant material
The fruit (Opuntia ficus) was purchased from Kovai Pazhamudir Nilayam, Velachery, Chennai, Tamil Nadu and were authentically identified by Prof. P. Jayaraman, Institute of Herbal Botany, Plant anatomy research centre, West Tambaram, Chennai, Tamil Nadu, India.
Processing of medicinal plants
The diseased free fruits were used to prepare extracts for the study. The fruits were collected and washed with running water and allowed it to remove the soil and dust particles. Then they were shade dried at room temperature for twenty days and then the dried plant materials were powdered using kitchen blender.
Preparation of plant extract
Extraction of Opuntia ficus fruit using different solvent was done according to the method of Medhe et al. [8]. The aqueous, chloroform, ethyl acetate, hexane and methanol extracts of fruits were prepared by dissolving 100gm of fine powdered fruit material.
The contents were kept in orbiter shaker for 48 hrs. Then the extracts were filtered and it is dried in hot air oven at 37ºC. Then the extract was stored under refrigeration at 4ºC for further studies.
In vitro Antioxidant potential of the whole plant extracts DPPH Assay
The antioxidant potential of the extracts was determined by 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity by the modified method of McCune and Johns [9].
Statistical analysis
The data of DPPH assays were subjected to statistical analysis and the Mean and SE for five individual observations was calculated. The significance of the sample mean was tested by Two Way ANOVA using SPSS software. The differences were considered as significant at p<0.05 level.
Results and Discussion
Yield of fruit extracts
The yield of Opuntia ficus extracts was maximum in methanol (2.5%), followed by chloroform (1.5%), hexane (1.0%), ethyl acetate (1.5%) and aqueous (1.5%). The colour of extracts was dark brown and the consistency was paste (Table 1).
| S. No. | Solvents | Weight of dried extract (g) | Yield (%) | Colour | Consistency |
|---|---|---|---|---|---|
| 1 | Aqueous | 100 | 1.5 | Dark brown | Paste |
| 2 | Chloroform | 100 | 1.5 | Dark brown | Paste |
| 3 | Ethyl acetate | 100 | 1.5 | Dark brown | Paste |
| 4 | Hexane | 100 | 1 | Dark brown | Paste |
| 5 | Methanol | 100 | 2.5 | Dark brown | Paste |
Table 1: Yield of solvent extracts of Opuntia ficus.
Antioxidant potential of Opuntia ficus extracts
The data on per cent inhibition of DPPH by aqueous, chloroform, ethyl acetate, hexane and methanol and along with L-Ascorbic acid (standard) is presented in (Table 2). The results depicted a dose-dependent inhibition in DPPH activity in all the extracts as well as in L-Ascorbic acid. The inhibition being higher in methanol extract than the other solvent extract. Ascorbic acid also better activity than methanol extract this may due to the antioxidative nature.
| S. No. | Concentration of extract (µg/ml) | % Inhibition | |||||
|---|---|---|---|---|---|---|---|
| Aqueous | Methanol | Chloroform | Hexane | Ethylacetate | Ascorbic acid | ||
| 1 | 20 | 0.934 ± 0.002* (-7.249) | 0.702 ± 0.0005* (-30.046) | 0.810 ± 0.001* (-23.809) | 0.984 ± 0.00* (-2.2509) | 0.866 ± 0.002* (-22.470) | 0.881 ± 0.014* (-13.679) |
| 2 | 40 | 0.836 ± 0.002* (-16.981) | 0.628 ± 0.0005* (-37.417) | 0.705 ± 0.001* (-33.740) | 0.976 ± 0.0005* (-3.012) | 0.747 ± 0.002* (-33.094) | 0.75 ± 0.007* (-26.542) |
| 3 | 60 | 0.711 ± 0.001* (-29.361) | 0.545 ± 0.003* (-45.717) | 0.589 ± 0.007* (-44.642) | 0.909 ± 0.002* (-9.731) | 0.674 ± 0.003* (-39.659) | 0.604 ± 0.002* (-40.777) |
| 4 | 80 | 0.654 ± 0.002* (-35.021) | 0.406 ± 0.003* (-59.528) | 0.448 ± 0.003* (-57.894) | 0.844 ± 0.003* (-16.120) | 0.524 ± 0.003* (-53.028) | 0.403 ± 0.002* (-60.463) |
| 5 | 100 | 0.591 ± 0.002* (-41.277) | 0.304 ± 0.004* (-69.721) | 0.282 ± 0.003* (-73.433) | 0.823 ± 0.002* (-18.205) | 0.476 ± 0.001* (-57.356) | 0.190 ± 0.007* (-81.325) |
| 6 | IC50 | 127.88 | 66.201 | 68.085 | 250.078 | 75.468 | 69.369 |
| Values are mean + S.E. of five individual observations. | |||||||
| Values in parentheses are per cent change over control. | |||||||
| Denotes per cent decrease over control. | |||||||
| *Values are significant at P<0.05. | |||||||
Table 2: DPPH activity of Opuntia ficus extracts.
The IC50 Opuntia ficus extracts found to the following order:
Methanol extract (66.20 μg/ml) ˂ chloroform extract (68.085 μg/ml) < ethyl acetate (75.468 μg/ml) ˂ Aqueous extract (127.88 μg/ml) ˂ Hexane (250.078 μg/ml).
The results indicate that methanol extract possess higher scavenging activity when compared to other solvent extract as shown in the (Figure 1).
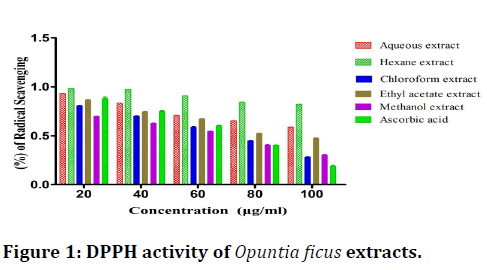
Figure 1. DPPH activity of Opuntia ficus extracts.
The DPPH results indicate that methanol extract showed more antioxidant potential than the other solvent extract and in future, the methanol can be used as drug for combating various ailments and this observation was consistent with earlier reports of Sannigrahi et al. [10] antioxidant potential of Enhydra fluctuans, Sathisha et al. [11] antioxidant potential of Curcuma longa, Caffea arabica, Tribulus terrestris and Bacopa monnieriand, Fatima et al. [12] using leaves and fruit extracts of Physalis minima, Solanum nigrum, Withania somnifera, Datura inoxia and Kigelia africana, antioxidant activity of Moroccan Thymus satureioïdes extracts [13], antioxidant potential of Terminalia arjuna [14] and antioxidant activity of Phragmanthera capitata [15].
DPPH result is in conformity with the high content of polyphenol may be present in methanol extract. This study revealed the effect of different solvents on the extraction and DPPH activities of Opuntia ficus fruit extracts. From the result it can concluded that methanol would be the solvent of choice as it gave better yield than the rest solvents.
In recent past, rising interest in search for phytochemicals, possessing antioxidant properties has been observed due to adverse effects of synthetic antioxidants on human health. The present study suggests that methanolic extract of Opuntia ficus fruit have a potent antioxidant activity. The antioxidant effects in fruits are mainly due to the presence of polyphenolic compounds such as bioflavonoid, phenolic acids, tannins, coumarins, lignans, quinones, stilbens, biphenols and curcuminoids as opined by Dluya et al. [16]. The antioxidant activity of methanol extract is mainly due to polyphenol content having their reduction/oxidation properties, which allow them to act as oxidizing agents, hydrogen donor and acceptor and quenching of singlet oxygen as stated by Ezeifeka et al. [17].
Though the antioxidant activity of the methanol extract were slightly less than the methanol extract and this may be due to proton-donating ability and free radical scavenger might act as a primary antioxidant in the methanol extract. It is known from the available literature that only the bioflavonoids of certain molecular structure such as 15 carbon skeleton ring structure linked heterocyclic rings and positioning of hydroxyl groups determines the antioxidant properties as opined by Zhu et al. [18].
There were significance differences (P<0.05) among the extracts and the standard Ascorbic acid at different concentration (μg/ml) this may be due to the radical scavenging of the extracts and the antioxidant mechanism might have involved in it. Relating the antioxidant activity of extracts derived from the Opuntia ficus fruit used, Methanolic extract showed higher radical scavenging activity than the aqueous, chloroform, hexane and ethyl acetate extracts this may be due to varying polarity, dipole moment, dielectric constant, boiling point, freezing point and readily soluble. More over methanol has been used by various researchers due to low boiling point of just 65⸰C. So the extraction and concentration of bioactive compounds is easy by using soxhlet extraction [19].
The DPPH assay indicated that the methanol extract has the ability to scavenge free radicals and the capacity to decolorize DPPH the controls. This can, as well, be attributed to the presence of polyphenolic compounds.
On the other hand, the antioxidant activity of methanol extract has been attributed to various mechanisms in our study such as initiators of reactive oxygen species (OH⸰ and HOO⸰), chelating or sequestering organic compounds, free radical substation, free radical addition, intra molecular free radical reaction, free radical rearrangement reactions, homolysis, electron transfer, nucleophilic aromatic substitution, carbon-carbon coupling reactions and elimination reactions.
This antioxidant activity was correlated with its function to prevent cardiovascular disease [20], bacterial diseases [21], anti-inflammatory effects [22], cancer [23], Type 2 diabetes [24], liver diseases [25], stomach cancer [26], prostate cancer [27], lung cancer [28], gastric cancer [29], colorectal cancer [30] and cervical cancer [31].
During oxidative free radicals causes neurological diseases like Alzheimer’s and Dementias, cardiovascular diseases by clogging the arteries, inflammatory disorders such as rheumatoid arthritis, aging (hair loss, change in hair texture, graying hair and loss of skin elasticity), diabetics and cancer. All these free radical diseases controlled by consuming antioxidant rich in food substance such as Opuntia ficus fruit which normally available in the arid, semiarid and desert regions of the world.
Conclusion
Opuntia ficus fruit have used to treat a variety of diseases such as antioxidant, antimicrobial and anticancer agent when compared to its other parts such stem, roots, flowers and seeds. Very few peoples in the world might be aware this commonly available prickly pear fruit which is highly loaded with nutraceutical properties. Henceforth the methanolic extract Opuntia ficus fruit could as dietary supplement in food industry and ethno pharmacological uses. Antioxidant activity showed by Opuntia ficus methanol extract might be due to the active phytocompounds present in it.
References
- Abramovič H, Grobin B, Ulrih NP, et al. The methodology applied in DPPH, ABTS and folin-ciocalteau assays has a large influence on the determined antioxidant potential. Acta Chim Slov 2017; 64:491-499.
- Zlatić N, Jakovljević D, Stanković M. Temporal, plant part, and interpopulation variability of secondary metabolites and antioxidant activity of Inula helenium l. Plants 2019; 8:179.
- López V, Akerreta S, Casanova E, et al. In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum Nutr 2007; 62:151-155.
- Vayalil PK. Antioxidant and antimutagenic properties of aqueous extract of date fruit (Phoenix dactylifera L. Arecaceae). J Agric Food Chem 2002; 50:610-617.
- Reddy CV, Sreeramulu D, Raghunath M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res Int 2010; 43:285-288.
- Tarwadi K, Agte V. Antioxidant and micronutrient potential of common fruits available in the Indian subcontinent. Int J Food Sci Nutr 2007; 58:341-349.
- Sreeramulu D, Reddy CV, Raghunath M. Antioxidant activity of commonly consumed cereals, millets, pulses and legumes in India. Indian J Biochem Biophys 2009; 46:112-115.
- Medhe S, Bansal P, Srivastava MM. Enhanced antioxidant activity of gold nanoparticle embedded 3, 6-dihydroxyflavone: A combinational study. Appl Nanosci 2014; 4:153-161.
- McCune LM, Johns T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J Ethnopharmacol 2002; 82:197-205.
- Sannigrahi S, Mazuder UK, Pal DK, et al. Antioxidant potential of crude extract and different fractions of Enhydra fluctuans Lour. Iran J Pharm Res 2010; 9:75-82.
- Sathisha AD, Lingaraju HB, Prasad KS. Evaluation of antioxidant activity of medicinal plant extracts produced for commercial purpose. E J Chem 2011; 8:882-886.
- Fatima I, Hussain T, Rafay M, et al. Evaluation of antioxidant activity of leaves and fruits extracts of five medicinal plants. Pak J Pharm Sci 2017; 30:1625-1628.
- Labiad MH, Harhar H, Ghanimi A, et al. Phytochemical screening and antioxidant activity of Moroccan Thymus satureioïdes extracts. J Mater Environ Sci 2017; 8:2132-2139.
- Kumar V, Sharma N, Sourirajan A, et al. Comparative evaluation of antimicrobial and antioxidant potential of ethanolic extract and its fractions of bark and leaves of Terminalia arjuna from north-western Himalayas, India. J Tradit Complement Med 2018; 8:100-106.
- Ohikhena FU, Wintola OA, Afolayan AJ. Quantitative phytochemical constituents and antioxidant activities of the mistletoe, Phragmanthera capitata (sprengel) balle extracted with different solvents. Pharmacogn Res 2018; 10:16-23.
- Dluya T, Daniel D, Gaiuson Y. Comparative biochemical evaluation of leaf extracts of Ficus sycomorus and Piliostigma thonningii plant. J Med Plants Stud 2015; 3:32-37.
- Ezeifeka GO, Orji MU, Mbata TI, et al. Antimicrobial activities of Cajanus cajan, Garcinia kola and Xylopia aethiopica on pathogenic microorganisms. Biotechnol 2004; 3:41-43.
- Zhu W, Jia Q, Wang Y, et al. The anthocyanin cyanidin-3-O-β-glucoside, a flavonoid, increases hepatic glutathione synthesis and protects hepatocytes against reactive oxygen species during hyperglycemia: Involvement of a cAMP–PKA-dependent signaling pathway. Free Radic Biol Med 2012; 52:314-327.
- Sun C, Wu Z, Wang Z, et al. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid Based Complement Altern Med 2015.
- Vivekananthan DP, Penn MS, Sapp SK, et al. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003; 361:2017-2023.
- Ashurst PR. Chemistry and technology of soft drinks and fruit juices. John Wiley & Sons; 2016.
- Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr Opin Food Sci 2016; 8:33-42.
- Wang TY, Li Q, Bi KS. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J Pharma Sci 2018; 13:12-23.
- Unuofin JO, Lebelo SL. Antioxidant effects and mechanisms of medicinal plants and their bioactive compounds for the prevention and treatment of type 2 diabetes: An updated review. Oxid Med Cell Longev 2020; 1-36.
- Satapathy S, Das N, Bandyopadhyay D, et al. Effect of Tulsi (Ocimum sanctum Linn.) supplementation on metabolic parameters and liver enzymes in young overweight and obese subjects. Indian J Clin Biochem 2017; 32:357-363.
- Zickute J, Strumylaite L, Dregval L, et al. Vegetables and fruits and risk of stomach cancer. Medicina 2005; 41:733-740.
- Van den Brandt PA, Zeegers MP, Bode P, et al. Toenail selenium levels and the subsequent risk of prostate cancer: A prospective cohort study. Cancer Epidemiol Prev Biomark 2003; 12:866-871.
- Tominaga K, Saito Y, Mori K, et al. An evaluation of serum microelement concentrations in lung cancer and matched non-cancer patients to determine the risk of developing lung cancer: A preliminary study. Jpn J Clin Oncol 1992; 22:96-101.
- Takezaki T, Gao CM, Ding JH, et al. Comparative study of lifestyles of residents in high and low risk areas for gastric cancer in Jiangsu Province, China; with special reference to allium vegetables. J Epidemiol 1999; 9:297-305.
- Papaioannou D, Cooper KL, Carroll C, et al. Antioxidants in the chemoprevention of colorectal cancer and colorectal adenomas in the general population: a systematic review and meta-analysis. Colorectal disease. 2011; 13:1085-1099.
- Myung SK, Ju W, Kim SC, et al, Korean Meta-analysis (KORMA) Study Group. Vitamin or antioxidant intake (or serum level) and risk of cervical neoplasm: A meta-analysis. BJOG 2011; 118:1285-1291.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ashok K1*, Babu M1, Kalaiarasi V2, Muthukumaravel A2 and Padmapriya G3
1Department of Microbiology and Biotechnology, Faculty of Arts and Science College, BIST, BIHER, Chennai, Tamil Nadu, India2Department of MCA, Faculty of Arts and Science, BIST, BIHER, Chennai Tamil Nadu, India
3Department of Chemistry, Faculty of Arts and Science College, BIST, BIHER, Chennai Tamil Nadu, India
Citation: Ashok K, Babu M, Kalaiarasi V, Muthukumaravel A, Padmapriya G,Antioxidant Potential of Opuntia ficus Fruit Extracts, J Res Med Dent Sci, 2022, 10(1): 402-406
Received: 06-Dec-2021, Manuscript No. JRMDS-21-46613; , Pre QC No. JRMDS-21-46613 (PQ); Editor assigned: 08-Dec-2021, Pre QC No. JRMDS-21-46613 (PQ); Reviewed: 22-Dec-2021, QC No. JRMDS-21-46613; Revised: 27-Dec-2021, Manuscript No. JRMDS-21-46613 (R); Published: 03-Jan-2022
