Research - (2022) Volume 10, Issue 9
Anti-Proliferative Activity of Andrographis paniculata Whole Plant Extract on A549 Lung Cancer Cell Line
Yoshita Guntupalli, Raghunandhakumar S*, Ezhilarasan D and Lakshmi T
*Correspondence: Raghunandhakumar S, Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Science, Saveetha University, Chennai, India, Email:
Abstract
Introduction: Lung cancer is one of the most common forms of cancer in the world. Cases are increasing globally everyday mainly due to increased habits of tobacco smoking. Timely diagnosis of lung cancer is complicated and requires effective coordination between multiple disciplines. Treatment modalities require a more personalized approach as lung cancer is a heterogeneous disease influenced by multiple factors. Andrographis paniculata is a medicinal plant which is traditionally used for many centuries for various purposes like upper respiratory tract infections, viral infections, etc. It is widely used in the field of Unani and Ayurveda. Aim: The aim of our study is to find out whether Andrographis paniculata whole plant extract has anti-proliferative activity on A549 lung cancer cell line. Materials and Methods: The cells (1 × 105 cells per ml) were seeded in a 96 well microtiter plate (100 μl per well) with replications. Treatment was conducted for 24hrs with different concentrations (10, 20, 40, 80, 160, 320 μg/ml) of andrographolide. After incubation, 20 μl of 5 mg/ml MTT stock solution was added to each well and incubated for 4 h at 37 °C. The obtained formazan crystals were solubilized with DMSO and the absorbance was measured at 570 nm using a microplate reader (SpectraMax M5, Molecular Devices, USA). Cell viability (%) has been shown as a ratio of absorbance (A570) in treated cells to absorbance in control cells (0.1 % DMSO) (A570). The IC50 was calculated as the concentration of sample needed to reduce 50 % of the absorbance in comparison to the DMSO-treated control. Percentage of cell viability was calculated following the equation: Cell viability (%)=Absorbance of sample/ Absorbance of control X 100 Inhibition (%)=100- cell viability (%) Results and Discussion: Andrographis paniculata whole plant extract was found to have anti-proliferative activity against A549 lung cancer cell line. We observed that when the concentration of plant extract increases the rate of cell death also increases, which clearly shows the anticancer efficacy of the plant extract. Conclusion: Hence Andrographis paniculata can be used as an effective treatment against lung cancer. Further investigation at pre-clinical and clinical levels for establishing it as a potential agent for cancer therapy.
Keywords
Lung cancer, A549, Andrographis paniculata, Cytotoxic, Antiproliferative
Introduction
Lung cancer is the most common form of cancer in the world (12.3% of all cancers), with an estimated 1.2 million new cases in 2000. Tobacco smoking is the most important cause of lung cancers with 80%–90% arising in cigarette smokers [1]. Unfortunately, owing to the global increase in the number of smokers since 1980,2 the burden of lung cancer will likely continue to increase in the coming years primarily in developing countries, where high-quality cancer registry data are unavailable [2]. Though lung cancer is strongly associated with cigarette smoking there is a substantial minority of patients who have never smoked. In this case, the population has more distinct molecular markers and less established risk factors [3].
Lung cancer is relatively rare before the fifth decade of life; risk increases with age thereafter. Men are more affected than women. Coming into clinical manifestations, dyspnea is present in one-third to onehalf of patients, there is risk of developing pulmonary emboli, pneumothoraces, pleural effusions, and pericardial effusions, chest pain is a less common symptom, Para neoplastic syndromes, Lambert-Eaton myasthenic syndrome. Cerebellar ataxia, hypercalcemia, etc. [4]. Timely detection, diagnosis, and subsequent treatment for lung cancer is critical to patient outcomes and well- being. Delays in any part of the process, from initial evaluation and referral, to definitive diagnosis, treatment, follow-up, and survivorship care, may lead to adverse patient outcomes.
Timely lung cancer diagnosis and treatment requires quick and effective coordination and communication across multiple disciplines—e.g., primary care, radiology, pulmonology, medical oncology, radiation oncology, and surgery [5]. Lung cancer is a very heterogeneous disease, at a cellular and histological level. Diagnostic challenges are deeply related to the development of personalized therapy and molecular and precise histological characterizations of lung cancer [6]. Targeted therapy and immunotherapy for lung cancer include targeting of epidermal growth factor mutations, EML4-ALK Translocations, ROS1 rearrangements, BRAF V600 mutations, MET Amplification, RET rearrangements, HER2 mutations, etc. [7].
Andrographis paniculata (AP) is a medicinal plant traditionally used as an anti-inflammatory and antibacterial herb. Andrographolide, the major active component of A. paniculata, exhibits diverse pharmacological activities, including anti-inflammation, anti- cancer, anti-obesity, anti-diabetes, and other activities [8]. In the Unani and Ayurvedic medicines, AP is one of the most used medicinal plants. It is commonly called ‘Nilavembu’. The balancing of proinflammatory and anti-inflammatory cytokines is the result of antiinflammatory performance of andrographolide [9]. AP has been found to counteract interference with the cell cycle. Such interference is the basis for the development of cancer or infection with viruses such as HIV-1. Andrographolide are thought to enhance immune system functions such as production of white blood cells (scavengers of bacteria and other foreign matter), release of interferon, and activity of the lymph system [10]. Our team has extensive knowledge and research experience that has translate into high quality publications [11-30].
The aim of this study is to find the anti-proliferative activity of Andrographis paniculata whole plant extract on A549 lung cancer cell line.
Materials and Methods
Chemicals
DMEM medium, 0.25% Trypsin-EDTA solution, sodium bicarbonate solution, bovine serum albumin (BSA), low melting agarose, MTT from Sigma Chemicals Co., St. Louis, USA. fetal bovine serum (FBS) and antibiotic/ antimycotic solution, DMSO were from Himedia, Sodium phosphate monobasic and dibasic, sodium chloride, sodium hydroxide, sodium carbonate, hydrochloric acid and methanol were purchased from Sisco Research Laboratories (SRL) India.
Preparation of the herbal extract
Stem powder of Andrographis paniculata (AP) was obtained from IMPCOPS (Chennai, India) and used for the present study. About 50 g of AP powder was soaked in 500 mL of aqueous and kept for 3 days in a static condition at room temperature. The solution was then filtered with crude filter paper followed by Whattman paper. Fine filtrate was subjected to rota evaporation after that 3g of the material was obtained. The total ethanol extract was concentrated in a vacuum evaporate and immediately stored at 4 ̊C.
Cell culture reagents dulbecco's modified eagle's medium (DMEM)
Commercially available DMEM contains 7.5% sodium bicarbonate solution. 5ml of penicillin/streptomycin solution and 0.5ml of amphotericin B solution were added to 500ml of DMEM. The medium was then placed inside the hood and sterile filtered (0.22μ). The medium was dispensed into sterile containers and stored at 4°C.
Growth medium [DMEM with 10% Fetal Bovine Serum (FBS)]
10 ml of FBS was made up to 100ml using sterile DMEM. It was stored in a sterile container in cool and aseptic condition.
Phosphate buffered saline (PBS; pH 7.4)
0.63 g of sodium phosphate monobasic (NaH2PO4), 0.17 g of sodium phosphate dibasic (Na2HPO4) and 4.5 g of sodium chloride (NaCl) were dissolved in 500 ml of double autoclaved milli Q water. Using 1 N HCl and 1 N NaOH, the pH was adjusted to 7.4, sterile filtered (0.22 μ) and then stored in a sterile container.
Trypsin-EDTA
Trypsin was purchased as 1 x with EDTA (0.5% trypsin, 5.3 mm EDTA sodium salt). (Note: Freeze-thaw process does not affect the enzyme activity. Thawing is done at room temperature).
Cell line
Human lung adenocarcinoma-A549 cell lines were obtained from the National Centre for Cell Science (NCCS, Pune), India. The cells were cultivated in T255 culture flasks which contained DMEM medium supplemented with 10% FBS. Upon reaching confluence, the cells were detached using Trypsin-EDTA solution.
Cell proliferation (MTT) assay
The proliferation of A549 cells were evaluated by MTT assay Koka et al., 2018(31).The anticancer activity of different concentrations of Andrographis paniculata whole plant extract on A549 were determined by the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) assay to assess the cytotoxicity according to the method described by Koka, et al. For the assay, cells were plated in 96-well plates at the density of 5x103/100μl. The cells were incubated for 24 hrs. The cells were then treated with AP extract at increasing concentrations of 10, 20, 40, 80, 160 and 320μg/ml. Wells with extract free mediums were used as negative controls. After 24 hrs. of incubation at 37°C , 10μl MTT reagent was added to each well and incubated for 4 hrs. in dark at 37°C. 100μl of Sorenson glycine buffer comprising of 0.1M glycine, 0.1M NaCl, pH 10.5 with 0.1N NaOH was added to the wells in order to solubilize the formazan crystals formed by the viable cells. The absorbance was then measured at 570 nm. The experiment was repeated thrice and each concentration of extract was tested in triplicates. The percentage of cell viability and inhibition percentage were calculated.
Cell viability (%)=Absorbance of sample/Absorbance of control X 100
Inhibition (%)=100-Cell Viability (%)
Statistical analysis
All data obtained were analyzed by Student-t-test using MS-Excel, represented as mean ± SD for six animals in each group. The results were computed statistically (SPSS/10 Software Package; SPSS Inc., Chicago, IL, USA) using one-way ANOVA. Post-hoc testing was performed for inter comparisons using the LSD. In all tests, the level of statistical significance was set at p<0.05.
Results and Discussion
From Figure 1 we observed that the dose dependent manner Andrographis paniculata whole plant extract treatment significantly decreases the cell viability in dose and time dependent manner. The anti-proliferative activity of A. paniculata in lung cancer cells at 20μg/ml after 24hrs of incubation has inhibited the 50% of cell proliferation, and it has been considered as IC50 value respectively.
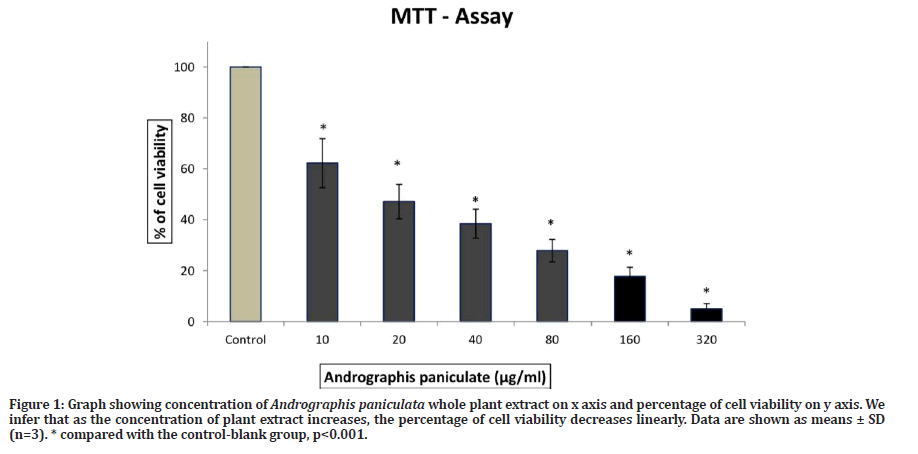
Figure 1: Graph showing concentration of Andrographis paniculata whole plant extract on x axis and percentage of cell viability on y axis. We infer that as the concentration of plant extract increases, the percentage of cell viability decreases linearly. Data are shown as means ± SD (n=3). * compared with the control-blank group, p<0.001.
Uncontrolled proliferation of cells due to loss of cell cycle control pathways leads to cancer. Cancer cells display much higher rates of proliferation than normal cells. Some currently used antitumor drugs, such as vinca alkaloids and taxanes, act by targeting microtubules and inhibiting mitosis. However these inhibitors have still not been approved for use in chemotherapy regimens [31,32]. Chemotherapy administration may result in the disruption of circadian rhythms and impairment of quality of life (QoL) of cancer patients [33].
Medicinal plants are an integral part of human life to combat the sufferings from the dawn of civilization. It is estimated that more than 80,000 of total plant species have been identified and used as medicinal plants around the world. Andrographis paniculata (AP) is an important medicinal plant and widely used around the world. It belongs to the family Acanthaceae. Its extracts contain diterpenoids, diterpene glycosides, lactones, flavonoids, and flavonoid glycosides [9]. It was found that the aqueous extract showed significant antimicrobial activity, which may be due to the combined effect of the isolated arabinogalactan proteins and andrographolide [34]. Andrographolide showed anticancer activity on diverse cancer cells representing different types of human cancers [35]. The compound exerts direct anticancer activity on cancer cells by cell‐cycle arrest at G0/G1 phase through induction of cell‐cycle inhibitory protein p27 and decreased expression of cyclin‐dependent kinase 4 (CDK4) [36]. Andrographolide and its derivatives have been shown to have anti‐inflammatory effects in experimental models of asthma, stroke and arthritis, as well as in patients with upper respiratory tract infections. Andrographolide reduces the production of cytokines, chemokines, adhesion molecules, nitric oxide and lipid mediators, probably via inhibition of the nuclear factor (NF)‐κB signaling pathway [37]. Andro induced apoptosis in human cancer cells via activation of caspase 8 in the extrinsic death receptor pathway and subsequently with the participation of mitochondria [38]. Andro triggered a caspase 8-dependent Bid cleavage, followed by a series of sequential events including Bax conformational change and mitochondrial translocation, cytochrome c release from mitochondria, and activation of caspase 9 and 3. Hence, it was found that pro-apoptotic Bcl-2 x.
In recent years, pharmaceutical chemists have synthesized numerous andrographolide derivatives, which exhibit essential pharmacological activities such as those that are anti-inflammatory, antibacterial, antitumor, antidiabetic, anti-HIV, antifeedant, and antiviral [38,40]. In a radiation therapy study, andrographolide was found to sensitize Ras-transformed cells and significantly delay tumor growth. Experimental evidence suggests that andrographolide attenuates endothelial cell motility and tumor-endothelial cell interaction [39,41].
From our results Andrographis paniculata whole plant extract induces apoptosis thereby inhibiting the cell proliferation against lung cancer cell lines. It was found that the apoptotic activity was dose dependent and cell viability has reduced accordingly at the concentration of 20μg/ml 50% of the cell proliferation has inhibited for 24hrs.
Conclusion
The present study clearly showed that the AP whole plant extract has enhanced antiproliferative activity in A549 lung cancer cell lines. Within the limitations of this study it was found that Andrographis paniculata whole plant extract was cytotoxic and it inhibited the cell proliferation against A459 lung cancer cell line. With further research on AP we can find out whether it can be used as an early and effective natural treatment for lung cancer in future medicine. However, the in vivo study using an experimental cancer animal model needed to prove a potential anticancer effect of Andrographis paniculata.
Acknowledgement
We thank Saveetha Institute of Medical and Technical Sciences for providing us the support to conduct the study.
Conflict of Interest
The author declares that there was no conflict of interest in the present study.
Source of Funding
The study was funded by the following agencies
Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Science, Saveetha University, Chennai, India.
Sarkav Health Services.
References
- Minna JD, Roth JA, Gazdar AF. Focus on lung cancer. Cancer Cell 2002; 1:49–52.
- Bade BC, Dela Cruz CS. Lung cancer 2020: Epidemiology, etiology, and prevention. Clin Chest Med 2020; 41:1–24.
- Rivera GA, Wakelee H. Lung cancer in never smokers. Adv Exp Med Biol 2016; 893:43–57.
- Nasim F, Sabath BF, Eapen GA. Lung cancer. Med Clin North Am 2019; 103:463–473.
- Hussain S. Nanomedicine for treatment of lung cancer. Adv Exp Med Biol 2016; 890:137–147.
- de Sousa VML, Carvalho L. Heterogeneity in lung cancer. Pathobiology 2018; 85:96–107.
- Paramasivam A, Raghunandhakumar S, Priyadharsini JV, et al. In vitro anti-neuroblastoma activity of thymoquinone against neuro-2a cells via cell-cycle arrest. Asian Pac J Cancer Prev 2015; 16:8313–8319.
- Dai Y, Chen SR, Chai L, et al. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Critical Rev Food Sci Nutr 2019; 59:17-29.
- Hossain MD, Urbi Z, Sule A, Rahman KM. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Scientific World J 2014; 2014.
- Tandon C, Mathur P, Sen M. Andrographis paniculata Nees (Kalmegh): A review on its antibacterial activities and phytocompounds. Eur J Med Plants 2015; 8:1.
- Rajeshkumar S, Kumar SV, Ramaiah A, et al. Biosynthesis of zinc oxide nanoparticles usingMangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb Technol 2018; 117:91–95.
- Nandhini NT, Rajeshkumar S, Mythili S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal Agric Biotechnol 2019; 19:101138.
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp.–mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ Sci Pollut Res 2020; 27:8166–8175.
- Rajkumar PV, Prakasam A, Rajeshkumar S, et al. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. South African J Chem Eng 2020; 32:1-4.
- Rajasekaran S, Damodharan D, Gopal K, et al. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel 2020; 277:118166.
- Santhoshkumar J, Sowmya B, Venkat Kumar S, et al. Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea. S Afr J Chem Eng 2019; 29:17–23.
- Raj RK. β‐Sitosterol‐assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J Biomed Materials Res 2020; 108:1899-908.
- Saravanan M, Arokiyaraj S, Lakshmi T, et al. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog 2018; 117:68–72.
- Gheena S, Ezhilarasan D. Syringic acid triggers reactive oxygen species–mediated cytotoxicity in HepG2 cells. Hum Exp Toxicol 2019; 38:694–702.
- Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int 2018; 17:192–197.
- Ezhilarasan D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab J Gastroenterol 2018; 19:56–64.
- Gomathi AC, Xavier Rajarathinam SR, Mohammed Sadiq A, et al. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J Drug Deliv Sci Technol 2020; 55:101376.
- Dua K, Wadhwa R, Singhvi G, et al. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev Res 2019; 80:714–730.
- Ramesh A, Varghese S, Jayakumar ND, et al. Comparative estimation of sulfiredoxin levels between chronic periodontitis and healthy patients - A case-control study. J Periodontol 2018; 89:1241–1248.
- Arumugam P, George R, Jayaseelan VP. Aberrations of m6A regulators are associated with tumorigenesis and metastasis in head and neck squamous cell carcinoma. Arch Oral Biol 2021; 122:105030.
- Joseph B, Prasanth CS. Is photodynamic therapy a viable antiviral weapon against COVID-19 in dentistry? Oral Surg Oral Med Oral Pathol Oral Radiol 2021; 132:118.
- Ezhilarasan D, Apoorva VS, Ashok Vardhan N. Syzygium cumini extract induced reactive oxygen species-mediated apoptosis in human oral squamous carcinoma cells. J Oral Pathol Med 2019; 48:115–121.
- Duraisamy R, Krishnan CS, Ramasubramanian H, et al. Compatibility of nonoriginal abutments with implants: evaluation of microgap at the implant-abutment interface, with original and nonoriginal abutments. Implant Dent 2019; 28:289–295.
- Gnanavel V, Roopan SM, Rajeshkumar S. Aquaculture: An overview of chemical ecology of seaweeds (food species) in natural products. Aquaculture 2019; 507:1–6.
- Markov A, Thangavelu L, Aravindhan S, et al. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther 2021; 12:192.
- Koka P, Mundre RS, Rangarajan R, et al. Uncoupling warburg effect and stemness in CD133 cancer stem cells from Saos-2 (osteosarcoma) cell line under hypoxia. Mol Biol Rep 2018; 45:1653–1662.
- Marzo I, Naval J. Antimitotic drugs in cancer chemotherapy: promises and pitfalls. Biochem Pharmacol. 2013 Sep 15;86(6):703–10.
- Sultan A, Pati AK, Choudhary V, et al. Hospitalization-induced exacerbation of the ill effects of chemotherapy on rest-activity rhythm and quality of life of breast cancer patients: A prospective and comparative cross-sectional follow-up study. Chronobiol Int 2018; 35:1513.
- Singha PK, Roy S, Dey S. Antimicrobial activity of Andrographis paniculata. Fitoterapia 2003; 74:692.
- Kumar RA, Sridevi K, Kumar NV, et al. Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol 2004; 92:291-295.
- Raghunandhakumar S, Paramasivam A, Senthilraja S, et al. Thymoquinone inhibits cell proliferation through regulation of G1/S phase cell cycle transition in N-nitrosodiethylamine-induced experimental rat hepatocellular carcinoma. Toxicol Lett 2013; 223:60–72.
- Lim JCW, Chan TK, Ng DSW, et al. Andrographolide and its analogues: versatile bioactive molecules for combating inflammation and cancer. Clin Exp Pharmacol Physiol 2012; 39:300–310.
- Asokkumar S, Naveenkumar C, Raghunandhakumar S, et al. Antiproliferative and antioxidant potential of beta-ionone against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Mol Cell Biochem 2012; 363:335–345.
- Zhou J, Zhang S, Choon-Nam O, et al. Critical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem Pharmacol 2006; 72:132-144.
- Anandakumar P, Kamaraj S, Jagan S, et al. Capsaicin inhibits benzo(a)pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflammation Res 2012; 61:1169–1175.
- Jayakumar T, Hsieh CY, Lee JJ, et al. Experimental and clinical pharmacology of Andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Alternat Med 2013; 2013:846740.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Yoshita Guntupalli, Raghunandhakumar S*, Ezhilarasan D and Lakshmi T
Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Science, Saveetha University, Chennai, IndiaReceived: 20-Aug-2022, Manuscript No. jrmds-22-70107; , Pre QC No. jrmds-22-70107(PQ); Editor assigned: 22-Aug-2022, Pre QC No. jrmds-22-70107(PQ); Reviewed: 13-Sep-2022, QC No. jrmds-22-70107(Q); Revised: 16-Sep-2022, Manuscript No. jrmds-22-70107(R); Published: 23-Sep-2022
