Research Article - (2025) Volume 13, Issue 4
Assessment of Coating Zirconium implant material with Nanoparticles of Faujasite
Ahmed Ali Mohammed1*, Thekra Ismail Hamad2 and Ahmed Hameed Neamah3
*Correspondence: Ahmed Ali Mohammed, Department of Dental Technology, College of Medical Technology, Al-Farahidi University, Baghdad, Iraq, Email:
Abstract
Aim: To evaluate the wettability and micro hardness of Zirconium (ZrO2) dental material when coated with different concentrations of Faujasite.
Materials and methods: 30 circular disks produced from ZrO2, then each group classified into 10 control groups, 10 coated groups with 3% Faujasite and 10 coated groups with 7% faujasite by electro spun tool to study variable properties in hardness and water contact angle of implant materials.
Results: This study stated the high hardness in 7% of faujasite concentration for ZrO2, in addition, the contact angle decreased gradually until reach 0° in 7% concentration of faujasite with ZrO2.
Conclusion: Water Contact Angle (WCA) declined till disappeared in (7% wt.) of faujasite coated with the ZrO2 group, also in the same group the microhardness became high compared with other groups due to alteration in surface morphology of substrate and properties of coated material.
Keywords
Electrospinning, Microhardness, Wettability, Circular disk, Polyvinylpyrrolidone (PVP), Water contact angle
Introduction
Titanium was used as a dental material for several decades and considered as standardization of dental implants but because of disadvantages such as galvanic corrosion and cellular sensitivity associated with the saliva of humans [1]. It was an alternative with ceramic dental material such as zirconia. Zircon strong transition metal, grey-white and lustrous named zirconium. It was the oxide form of zircon [2].
In 1824 Jones Jakob Berzelius was the first to produce zirconium in the form of impure. In dentistry, it was utilized to fabricate esthetic orthodontic brackets, crown/bridge, endodontic posts, implant abutments for rehabilitation of partial and complete arches and restorations. Faujasite was classified into Y had a Si/Al ratio of more than 1.5 and X had a Si/Al from (1-1.5). Zeolitic materials were characterized mainly by their ion exchange and adsorption capacities. In addition, they can be produced in the laboratory using low, cost raw materials. These materials have been widely used as adsorbents, molecular sieves and ion exchangers in the treatment of wastewater, air purifiers, catalysts and catalyst support [3]. The hardest of ceramic was a Zirconia. It was widely produced in a monolithic phase for many clinical usages, was known as Yttria-Stabilized Tetragonal Zirconia Polycrystal (Y-TZP). Different types of Y-TZP were found can be based on heat treatment, sintering, dopants and additives [4]. Absent of toxicity and good mechanical properties of Y-TZP were forced to choose it in dental usage, though it has one problem about matching it with natural teeth to improve esthetic [5].
Electro spinning was one way to produce filaments (ultrafine fiber) from many types of materials like composite, polymer and ceramic. The machine was composed of three parts, conductive collector, syringe with metallic needle and voltage power supply. This process was divided into various techniques such as bubble electro spinning, siro-electro spinning, vibration electro spinning and magneto-electro spinning [6]. Various devices were utilized to analyze surface texture and surface properties such as Water Contact Angle (WCA) and micro hardness by the x-ray diffraction and energy diffraction spectroscopy.
Materials and Methods
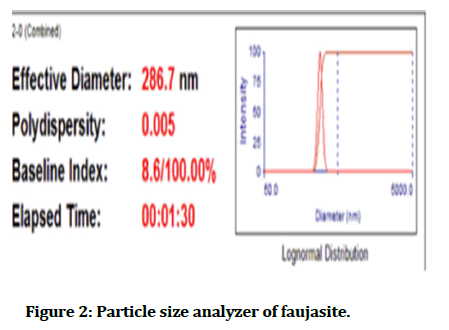
Partially sintered zirconia from VITA supplied in block disc form with a dimension of 98.4 mm in diameter and 12 mm in height, the white shade (A1), was cut into small circular discs (10 mm diameter and 1 mm thickness) as shown in Figure 1 the substrates prepared with exo-cad dental CAD Software [7]. To prepare zirconia samples, fractional sintered zirconia block disc cut according to the selected measurements utilizing a dental computerized three-axis milling system, Computer Aided Design/Computer Aided Manufacturing (CAD/CAM) imes-icore, Germany). Substrates sintered in sintering heater of imes-icore up to 1650°C, according to manufacture instruction. Then substrates cleaned ultrasonically in ethanol for half an hour and then put aside in the air before coating [8]. Polyvinylpyrrolidone (PVP) (MW: 40,000) produced by alpha chemical (made in India), PVP, and distilled water was considered as a solvent for the faujasite manufactured by China with a particle size of about 286.7 as shown in the Figure 2 and measurements of parameters device mentioned in Table 1. The sample of zirconia coated with faujasite by using the electrospun technique as appeared in Figure 3.
Figure 1: Zirconium disk.
By using an electrospinning machine (made in the USA) filaments was formed after mixing 40% wt. of PVP with concertation of faujasite (3% and 7%) and addition D.W to become the mixture solution 5 gm for each percentage, the parameters of the machine were 20 K.V. of the voltage supply, flow rate (1.5 ml/h) and the distance (13 cm) between detector with samples and head of needle syringe found in the electrospun machine after the filaments were formed as shown in Figure 3, all samples kept in room temperature to evaporate the solvent [9].
Figure 2: Particle size analyzer of faujasite.
| Measurement parameter | |||
|---|---|---|---|
| Temperature | 25.0°C | Runs completed | 3 |
| Liquid | water | Burn duration | 00:00:30 |
| Viscosity | 0.890 cp | Total elapsed time | 00:01:30 |
| Ref. index fluid | 1.330 | Average count rate | 450.1 Keps |
| Angle | 90.00 | Ref. index real | 1.590 |
| Wavelength | 660.00 | Ref. index image | 0.000 |
| Baseline | Auto (slope analysis) | Dust filter setting | 30.00 |
Table 1: Measurement parameter of particle size analyzer device.
| Elt | Line | Int | Error | K | Kr | W% | A% | ZAF |
|---|---|---|---|---|---|---|---|---|
| C | Ka | 73.5 | 228.5689 | 0.1726 | 0.0734 | 22.25 | 29.34 | 0.3298 |
| O | Ka | 512.1 | 228.5689 | 0.5986 | 0.2545 | 57.49 | 56.93 | 0.4426 |
| Na | Ka | 434.1 | 83.6938 | 0.1938 | 0.0824 | 17.86 | 12.30 | 0.4615 |
| Mg | Ka | 30.7 | 83.6938 | 0.0128 | 0.0054 | 1.11 | 0.72 | 0.4908 |
| Si | Ka | 28.5 | 23.5762 | 0.0122 | 0.0052 | 0.73 | 0.41 | 0.7111 |
| P | Ka | 20.5 | 23.5762 | 0.0100 | 0.0042 | 0.56 | 0.29 | 0.7525 |
| 1.0000 | 0.4251 | 100.00 | 100.00 |
Table 2: Quantitive results of uncoated group.
Characterization
Nanofibers characterized by the Energy Diffraction Spectroscopy (EDS) and X-Ray Diffraction (XRD) in order to evaluate water contact angle and micro-hardness for various percentage coating of faujasite and compared with un-coating group.
Figure 3: Samples coating with faujasite suspension inside electrospinning machine.
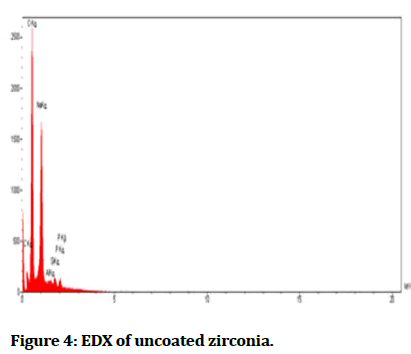
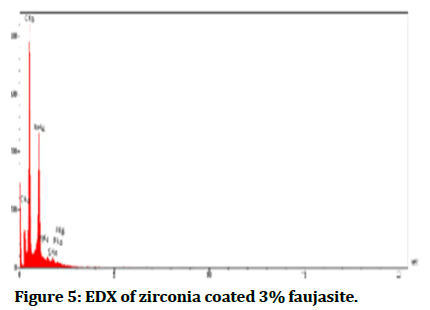
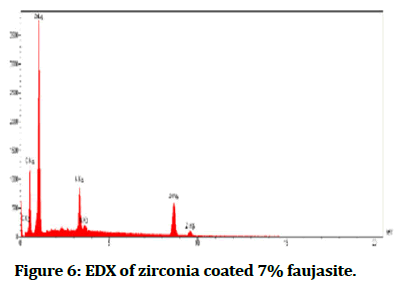
The Energy Diffraction Spectroscopy (EDS): It was a method to determine element composition material which formed samples and then analysed by forming peaks for each ingredient as shown in Figures 4-6 interprets EDX for each percentage of faujasite (3% and 7%) in respectively, that utilized in the coating of zirconia and compared it with the control group as seen in Figure 4. Also, Tables 2-4 showed the quantities results for all groups control, coated 3% and coated 7% of faujasite in respectively.
Figure 4: EDX of uncoated zirconia.
Figure 5: EDX of zirconia coated 3% faujasite.
| Elt | Line | Int | Error | K | Kr | W% | A% | ZAF |
|---|---|---|---|---|---|---|---|---|
| C | Ka | 41.5 | 253.7275 | 0.1049 | 0.0388 | 15.87 | 21.5 | 0.2442 |
| O | Ka | 636 | 253.7275 | 0.6807 | 0.2515 | 62.48 | 63.56 | 0.4025 |
| Na | Ka | 483.6 | 304.1865 | 0.17 | 0.0628 | 18.87 | 13.36 | 0.3328 |
| Al | Ka | 38.2 | 40.3406 | 0.0115 | 0.0042 | 0.89 | 0.54 | 0.4749 |
| Si | Ka | 47.4 | 40.3406 | 0.0145 | 0.0054 | 0.88 | 0.51 | 0.6067 |
| P | Ka | 54.3 | 40.3406 | 0.0184 | 0.0068 | 1 | 0.53 | 0.6776 |
| 1 | 0.3694 | 100 | 100 |
Table 3: Quantitive results of zirconia coated 3% faujasite.
| ELT | LINE | NIT | Error | K | KR | W% | A% | ZAF |
|---|---|---|---|---|---|---|---|---|
| C | KA | 5.2 | 15.3921 | 0.0114 | 0.0066 | 3.56 | 7.10 | 0.1850 |
| 0 | KA | 314.4 | 15.3921 | 0.2937 | 0.1696 | 49.33 | 73.78 | 0.3438 |
| K | KA | 285.1 | 0.8243 | 0. 1147 | 0.0662 | 7.61 | 4.66 | 0.8707 |
| Zn | KA | 351.8 | 1.3172 | 0.5801 | 0.3350 | 39.50 | 14.46 | 0.848 |
| 1.0000 | 0.5774 | 100.00 | 100.00 |
Table 4: Quantitive results of zirconia coated 7% faujasite.
Figure 6: EDX of zirconia coated 7% faujasite.
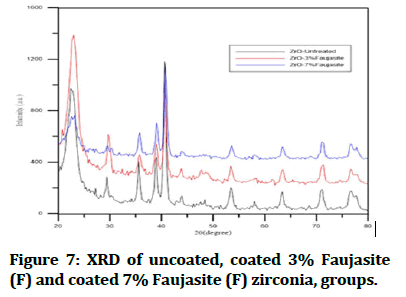
X-Ray Diffraction (XRD): An automated x-ray diffractometer was employed for phase analysis by using Cu-Kα radiation (λ=1.5406 Å), XRD-6000, SHIMADZU (Japan) the operation was done at 30 mA and 40 kV. Ambient laboratory temperature using 10 s/angular step (1 angular step=0.02°) was used for taken diffraction patterns. Depending on the Joint Committee on Powder Diffraction Standards (JCPDS) of the international centre for the diffraction data the peak indexing was carried out, Phase analysis was applied to the samples before coating surface structuring, and after coating technique for 3% and 7% concentration of faujasite beside to the control disks as seen in Figure 7 and basic data in the Table 5 showed the three strongest peaks in each group which interpreted amount of changing occurred by the effect of treatment with faujasite that included peak number, 2 theta degree, amount of diffraction, intensity, and full width at a high maximum of XRD profile.
Figure 7: XRD of uncoated, coated 3% Faujasite (F) and coated 7% Faujasite (F) zirconia, groups.
| Strongest 3 peaks | |||||
|---|---|---|---|---|---|
| Uncoated ZrO2 | |||||
| No. | Peaks no. | 2 theta (degree) | Diffraction (angstrom) | Intensity (I/I1) | FWHM |
| 1 | 3 | 30.1423 | 2.96248 | 100 | 0.5726 |
| 2 | 8 | 50.1979 | 1.81596 | 60 | 0.6307 |
| 3 | 5 | 34.7594 | 2.57882 | 29 | 0.3893 |
|
|
|||||
| 1 | 2 | 30.2361 | 2.95351 | 100 | 0.6756 |
| 2 | 5 | 50.3643 | 1.81035 | 54 | 0.6924 |
| 3 | 3 | 34.9005 | 2.56871 | 27 | 0.525 |
|
|
|||||
| 1 | 2 | 29.927 | 2.9833 | 100 | 0.8327 |
| 2 | 7 | 50.0502 | 1.82097 | 70 | 0.9082 |
| 3 | 8 | 59.2329 | 1.55871 | 43 | 1.0167 |
Table 5: Basic data of XRD for study groups.
Wettability analysis: In the department of chemical engineering/Al-technology university, Iraq. The measurements of wettability were done by goniometer CAM 110, made in Germany, to survey the effect of different percentages of faujasite coating with ZrO2 and evaluate wettability phenomena on selected disks 30 seconds was needed to capture an image after applying a drop of liquid on an intended surface, the procedure occurred at an ambient temperature.
Microhardness test: Digital Vickers micro-hardness tester Buehler micrometer 5103, USA, was used to record the micro-hardness of the ZrO2 disks coated by faujasite with 3% and 7%, and uncoated disks according to (ASTM E92-82, 1997), for 5 seconds 9.8 g load was applied to the surface of the disk by using Vickers indenter that joins optical microscopy. An average of 3 different readings was measured from the ten ZrO2 specimens for each selected concentration to compare between the control and coating groups.
Results
The mean water contact angle in ZrO2 with 7% faujasite was zero degree and for the ZrO2 with 3% faujasite was 21.46° but for control, ZrO2 was 77.72° and descriptive statistics of water contact angle test of the 3 groups were summarized in Table 6. The table shows the lowest water contact in group ZrO2 7% Faujasite (F) (0)° and the highest value of water contact in the control group of ZrO2 (78.65°).
| No. | Groups | No. of samples | Mean | S.D. | Minimum | Maximum |
|---|---|---|---|---|---|---|
| 1 | ZrO2 C | 10 | 77.72 | 0.579 | 76.65 | 78.65 |
| 2 | ZrO2 3% F | 10 | 21.461 | 1.049 | 20.121 | 22.876 |
| 3 | ZrO2 7% F | 10 | zero | zero | zero | zero |
Table 6: Descriptive statistic WCA for zirconia groups.
Statistically, the F-test of the one-way ANOVA test showed a non-significance difference in the water contact angle among the 3 groups, because of P>05 at three degrees of freedom as shown in Table 7.
| Source of variance | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Between groups | 1 | 1586 | 1586 | 1.969 | 0.233 |
| Within groups | 4 | 3222.3 | 805.575 | ||
| total | 5 | 4808.301 |
Table 7: One-way ANOVA test of WCA.
Three readings were obtained from each of the thirty specimens (ten discs for each group) by using the Vickers microhardness tester by applying 9.8 g load for 5 seconds descriptive statistics for micro-hardness was seen in the Table 8. The table shows the lowest mean value for group Control (C) of ZrO2 was 1275.38 H.V. and 1683.65 H.V. the highest mean was in group ZrO2 7% Faujasite (F).
| No. | Groups | No. of samples | Mean | S.D. | Maximum | Minimum |
|---|---|---|---|---|---|---|
| 1 | ZrO2 C | 10 | 1275.38 | 43.446 | 1190 | 1349 |
| 2 | ZrO2 3% F | 10 | 1646.4 | 87.398 | 1521 | 1763 |
| 3 | ZrO2 7% F | 10 | 1683.65 | 69.824 | 1587 | 1790 |
Table 8: Descriptive statistic of vickers microhardness test for zirconia groups.
ANOVA test was seen in Table 9 revealed that there was a highly significant difference among the groups P ≤ 01 degrees of freedom.
| Source of variation | DF | SS | MS | F | P |
|---|---|---|---|---|---|
| Between groups | 2 | 1019092.6 | 509546 | 106.14 | <.00853 |
| Residual | 27 | 129612.62 | 4800.4 | ||
| Total | 29 | 1148705 |
Table 9: One-Way ANOVA test for Vickers microhardness of Zirconia groups.
Discussion
In XRD analysis between study groups of ZrO2 as seen in Figure 7 and Table 5 especially at the intensity 100 (I/I1) showed a high difference in the full width at a high maximum of XRD profile (FWHM) which was responsible to describe surface and various material properties like plastic deformation, mechanical properties, and changed in microhardness, many surveys stated FWHM was an accurate signal of the surface work hardening compared with another micro-hardness testing. In this study, the results showed the (FWHM) at some intensity increased from 0.57 degrees in control groups and become in study groups coating 3% F 0.67 degree till reached to 0.83 degrees with a group of 7% F coated of ZrO2 [10].
In Table 4 compared with Tables 2 and 3 the EDX interpreted the present zinc clearly with study groups coated with 7% faujasite which was responsible to modify the mechanical and wettability properties, the addition of zinc to the Ti-6 Al-4 V result in low the modulus of elasticity and enhancement of micro-hardness, also Zinc (Zn) particles cause improvement in hydrophilicity and biocompatibility through cell proliferation and adhesion [11]. Due to found of oxygen, sodium, silicon, and aluminum in selected samples, so there were appeared in their low XPS resolution spectra. Faujasite has interpreted the crystallinity via EDS and FTIR analysis [12].
WCA decreased gradually with increased concentration of faujasite for dental implant materials until reached WCA=0° with coating 7% concentration of faujasite to ZrO2, interpreted increasing hydrophilicity of materials when coated with faujasite to improve dental implant properties, was significant with water absorption capability, it was a critical parameter in determent the liquid uptake from the media, Therefore, it can be considered as a significant indicator to evaluate the suitability of biomaterial for tissue engineering usages [13]. The θ angle for each scaffold was observed when a drop of water placed on the sample. For PLGA (poly lactic-co-glycolic acid/scaffold), θ=123.8° ± 5°, It shows that the structure of pure polymeric scaffold was hydrophobic for PLGA/zeolite 3 (wt.%), θ was declined to 101.83° ± 6°, while nano scaffold was stayed hydrophobic. Water contact angles for PLGA/zeolite 7 (wt.%) and PLGA/zeolite 10 (wt.%) were 94.64° ± 6° and 82.08° ± 4°, in sequence. This showed that adding more zeolite to PLGA made it more hydrophilic. These results observed that nanoparticle zeolite powder utilized in the composite had a high tendency to water wettability [14].
In zirconia groups showed enhancement in micro hardness values with coating material due to increase ingrain particle size and surface roughness caused bonding between coating materials and substrate of zirconia [15]. Other investigations may be demonstrated this variation by examination and study increase the micro hardness associated with magnifying grain size and diminishing surface roughness due to the load applied diminished upon micro crack adjacent to the penetration area of diamond tool [16].
Conclusion
This study clarified the micro hardness and Water Contact Angle (WCA) improved in ZrO2 groups by enhancement the concentration of nanoparticles of faujasite to develop dental implant material and restriction of implant failure in the future with advanced coated techniques.
References
- Safi IN, Hussein BMA, Al Shammari AM, et al. Implementation and characterization of coating pure titanium dental implant with sintered β-TCP by using Nd: YAG laser. Saudi Dent J 2019; 31.
[Crossref] [Google Scholar] [PubMed]
- Kim KT, Eo MY, Nguyen TTH, et al. General review of titanium toxicity. Int J Implant Dent 2019.
[Crossref] [Google Scholar] [PubMed]
- Burakov AE, Galunin EV, Burakova IV, et al. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review, ecotoxicology and environmental safety. Ecotoxicol Environ Saf 2018; 702-712.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Lawn BR. Novel zirconia materials in dentistry. J Dent Res 2017; 97:2.
[Crossref] [Google Scholar] [PubMed]
- Pieger S, Salman A, Bidra AS. Clinical outcomes of lithium disilicate single crowns and partial fixed dental prostheses: A systematic review. J Prosthet Dent 2014; 112:22-30.
[Crossref] [Google Scholar] [PubMed]
- Shao H, Fang J, Wang H, et al. Effect of electrospinning parameters and polymer concentrations on mechanical to electrical energy conversion of randomly oriented electrospun poly (vinylidene fluoride) nanofiber mats. RSC Adv 2015; 5.
- Rusli MSIC, Hassan MI, Sultana N, et al. Characterization of PCL/zeolite electrospun membrane for the removal of silver in drinking water. J Teknol 2017; 79:89-95.
- Safi IN, Hussein BMA, Al Shammari AM. Testing and characterization of sintered β-tricalcium phosphate coat upon zirconia dental implant using Nd: YAG laser. J Laser Appl 2019; 31.
- Anis SF, Hashaikeh R. Electrospun zeolite-y fibers: Fabrication and morphology analysis. Micropor Mesopor Mat 2016; 233:78–86.
- Vashista M, Paul S. Correlation between Full Width at Half Maximum (FWHM) of XRD peak with residual stress on ground surfaces. Philos Mag 2012; 92.
- Zhu C, Lv Y, Qian C, et al. Microstructures, mechanical and biological properties of a novel Ti-6 V-4 V/zinc surface nanocomposite prepared by friction stir processing. Int J Nanomed 2018; 13.
[Crossref] [Google Scholar] [PubMed]
- Zahmakiran M, Ozkar S. Zeolite confined nanostructured dinuclear ruthenium clusters: Preparation, characterization and catalytic properties in the aerobic oxidation of alcohols under mild conditions. J Mater Chem 2009; 19.
- Prasopdee T, Sinthuvanich C, Chollakup R, et al. The albumin/starch scaffold and its biocompatibility with living cells. Mater Today Commun 2021; 27.
- Davarpan R, Rafienia M, Salehi Rozve H, Karamian E, Sattary M. Fabrication and characterization of electrospun poly lactic-co-glycolic acid/zeolite nanocomposite scaffolds using bone tissue engineering. J Bioact Compat Polym 2018; 33:63-78.
- Lin SC, Lin WC, Hu TC, et al. Evaluation of the bonding strength between various dental zirconia models and human teeth for dental posts through in vitro aging tests. Coat 2021.
- Song N, Wang Z, Xing Y, et al. Evaluation of phase transformation and mechanical properties of metastable Yttria-Stabilized Zirconia by nanoindentation. Materials (Basel) 2019.
[Crossref] [Google Scholar] [PubMed]
Author Info
Ahmed Ali Mohammed1*, Thekra Ismail Hamad2 and Ahmed Hameed Neamah3
1Department of Dental Technology, College of Medical Technology, Al-Farahidi University, Baghdad, Iraq2Department of Prosthodontic, College of Dentistry, University of Baghdad, Baghdad, Iraq
3Department of Health Research, Al-Diwaniyah Secondary Dental Health Center, Al-Diwaniyah, Iraq
Citation: Ahmed Ali Mohammed, Thekra Ismail Hamad, Ahmed Hameed Neamah, Assessment of Coating Zirconium Implant Material with Nanoparticles of Faujasite, J Res Med Dent Sci, 2023, 11 (08): 006-012.
Received: 14-Jun-2022, Manuscript No. JRMDS-22-102454; , Pre QC No. JRMDS-22-102454 (PQ); Editor assigned: 16-Jun-2022, Pre QC No. JRMDS-22-102454 (PQ); Reviewed: 30-Jun-2022, QC No. JRMDS-22-102454; Revised: 14-Aug-2023, Manuscript No. JRMDS-22-102454 (R); Published: 21-Aug-2023