Research Article - (2022) Volume 10, Issue 10
Assessment of Serum and Salivary 14-3-3 ETA Protein in Rheumatoid Arthritis Patients
Ayoob Jasim Mohammed1*, Ameena Ryhan Diajil1 and Fedan Ihsan Hassan2
*Correspondence: Dr. Ayoob Jasim Mohammed, Department of Oral Medicine, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Aim: The present study aimed to assess the salivary level of eta protein as a proinflammatory marker in Rheumatoid Arthritis (RA) patients and correlate its level in the serum.
Materials and Methods: This research comprised 84 individuals with RA (69) and healthy individuals (15). Serum and salivary 14-3-3 eta protein levels and other clinical measures in serum subjected to laboratory tests including [C-Reactive Protein (CRP), Rheumatoid Factor (RF) and Anti Cyclical Citrullinated Peptide Antibody (ACCP)] using quantitative Enzyme- Linked Immune Sorbent Assay (ELISA) technique. Receiver-Operating Characteristic (ROC) curves analysis was employed to determine 14-3-3 eta protein sensitivity and specificity. The relationship between serum and salivary 14-3-3 eta protein in RA patients with other clinical measures was evaluated using spearman’s correlation.
Results: (Mean ± SD; Median) of salivary 14-3-3 eta protein in RA patients was (3.800 ± 2.659; 2.948) which was highly significant compared with healthy subjects (1.563 ± 0.620; 1.390; P<0.001). ROC was drawn to make comparison between RA patients with controls, showed a significant (P<0.001)
ROC curve showed that the AUC of 0.943 (95% confidence interval: 0.852-1.000). At a cutoff of 1.8 ng/mL, salivary 14-3-3 eta protein had a sensitivity of 98.5% and specificity of 80%. While the serum 14-3-3 eta protein at the same cut off had a sensitivity of 95.6% and specificity of 66.7%. In addition, salivary levels of 14-3-3 eta protein significantly and correlated with the serum level (r=0.302, P<0.012). Also, the results of the correlation revealed that serum levels of 14-3-3 eta protein was significantly correlated with serum ACCP (r=0.597, P<0.001), serum C-reactive protein (r=0.628, P<0.001), serum Rheumatoid Factor (RF) (r=0.518, P<0.001), and 28-joint Disease Activity Score (DAS) (r=0.286, P<0.017) in RA patients.
Conclusions: Both serum and salivary 14-3-3 eta protein is significantly increased in patient with rheumatoid arthritis.
Keywords
Salivary 14-3-3 ETA protein, Rheumatoid arthritis, DAS 28, Anti cyclical citrullinated peptide antibody, Rheumatoid factor
Introduction
Rheumatoid Arthritis (RA) is a chronic inflammatory autoimmune disease characterized by symmetrical small joints inflammation and affects approximate 1% of the population all over the world [1]. If RA patients left without treatment leads to severe joint destruction resulting in impaired physical function and disability in the workplace [2]. There are multiple autoantibodies that have been reported detectable in the serum of patients, which are associated with RA. Rheumatoid factors and anti-CCP antibodies have been shown to be useful diagnostic tools particularly in the early stages of the disease and predictive of disease progression [3]. Moreover, the Disease Activity Score 28 (DAS28) is used to assess the disease course and treatment outcome and is based on a count of 28 specified joints for swelling and tenderness [4]. However, the manifestations of RA are too diversified for physicians to distinguish those not that well differentiated from patients suffering from other diseases sharing common clinical features, resulting in many patients, earlier staged and/or serum-negative patients in particular, being miss or missed diagnosed. Therefore, researchers are working on finding novel diagnostic and predictive biomarkers with higher sensitivity and specificity to improve the diagnostic accuracy and disease outcome of RA [5].
The serum 14-3-3 eta protein is a unique biomarker to find RA disease [6]. Seven isoforms are known of the 14-3-3 intracellular protein family (β, γ, ε, eta, σ, θ, ζ). Easily detectable isoforms in synovial fluid and patient serum samples with inflammatory joint diseases are eta and γ [7]. 14-3-3 eta protein levels in serum of patients with RA higher than in those with primary sjogren's syndrome, osteoarthritis, gout, psoriasis, crohn's disease, multiple sclerosis, systemic lupus erythematosus, type 1 diabetes, ulcerative colitis, osteoporosis and scleroderma [2,8].
In this study, evaluation performance of salivary 14-3-3 eta protein levels investigated in patients with RA and healthy individuals to determine the sensitivity and specificity of salivary 14-3-3 eta protein obtained using a 14-3-3 eta protein quantitative Enzyme-Linked Immune Sorbent Assay (ELISA). Since serum 14-3-3 eta protein, ACCP and RF are often used to diagnose RA, the incremental benefit of adding salivary 14-3-3 eta protein to above diagnostic markers was also assessed in this study.
Materials and Methods
The patients were allocated from rheumatology consulting clinic in Baghdad teaching hospital. Demographic evaluation of all participants was done (Table 1). A total number of 84 subjects were included in this study, divided into two groups:
| Group I (n=15) | Group II (n=69) | p-value | |
|---|---|---|---|
| Gender (male:female) | 2:13 | 14:55 | 0.534 |
| Age (year) Median Inter-quarter (Q1-Q3) = 40 >40 |
43 40-45 6 9 |
47 40-45 19 50 |
0.339 |
| Duration of disease (year) <1 01\05\2022 >5 |
15 (21.8) 27 (39.1) 27 (39.1) |
||
| Status of Disease activity Active (relapse) Inactive (remission) |
59 (85.5) 10 (14.5) |
||
| DAS-28 Score Remission (n=10) Low (n=11) Intermediate (n=45) High (n=3) |
2.28 ± 0.16 3.00 ± 0.14 4.02 ± 0.49 5.92 ± 0.10 |
Table 1: Demographic and clinical characteristics of the participants.
• Group I (control group): The participant number was 15 (2 males and 12 females). Their median age was 43 years, with inter-quarter (Q1-Q3) 40-45, 6 of participants were ≤ 40 years and 9 of participants were >40 years.
• Group II (RA patients): The number of the participants was 69 (14 males and 55 females). Their median age was 47 years, with inter-quarter (Q1-Q3) 40-45, 19 of participants were ≤ 40 years and 50 of participants were >40 years.
There was no significant difference in age and gender between the two groups. The criteria of inclusion included: patients with newly diagnosed or known cases of RA patients. The criteria of exclusion included: diabetes mellitus patient, pregnancy and lactated mothers, malignancy, chronic liver disease, end-stage renal failure and other autoimmune diseases.
Sixty-nine patients with RA in this study were subjected according to criteria of American College of Rheumatology (ACR) in 2010 [6], then measurement of serum and salivary 14-3-3 eta protein levels were assessed. CRP, ACCP and RF were recorded in serum as well as clinical and demographic data were obtained. Ethical approval for all participants was obtained before the work.
Collection of saliva samples
From each participant the whole un-stimulated mixed (resting) saliva was obtained. A two-hour fast was required before collection. Individual rinsed their mouth with tap water. The saliva samples were collected into disposable containers. Then centrifuged (3000 rpm) for 10 min. The volume of each saliva sample was measured after separating the supernatants, then the samples were kept in an eppendorf tube and frozen at (-20°C) to be used for biochemical analysis.
The results expressed as number (%), median, interquartile, mean ± SD. P-value for categorized data was calculated by using Chi-squared test for comparison between Groups I and II Group I: control participants, Group II: rheumatoid arthritis patients, DAS: Disease Activity Score.
Immunoassays of serum 14-3-3 ETA protein, RF and ACCP and salivary 14-3-3 eta protein
Using ELISA kit supplied by bioassay technology laboratory (China) for measurement of 14-3-3 eta protein in serum and saliva. ACCP was measured by ELISA using reagents supplied by bioassay technology laboratory (England). Using ELISA kit supplied by CELL BIOLABS, INC for measurement of RF and CRP. ELISA tests were performed according to the manufacturer’s instructions.
Statistical analysis: The results expressed as number (%), median, inter-quartile, mean ± SD. P-value for categorized data was calculated by using Chi-squared test for comparison between Groups I and II Group I: control participants, Group II: rheumatoid arthritis patients, DAS: Disease Activity Score. The results of assessment of biomarkers related to rheumatoid arthritis compared with health subjects are expressed as mean ± SD (median). P-value was calculated using independent twosample t-test for continuous data. Receiver-Operating Characteristic (ROC) curves analysis was performed to determine the sensitivity and specificity of serum and salivary 14-3-3 eta protein. The relationship between serum and salivary 14-3-3 eta protein measured then the relationship of serum 14-3-3 eta protein and other clinical measures in patients with RA was assessed using spearman correlation. Statistical analysis was performed with SPSS software (version 24.0). P value <0.05 denoted statistical significance.
Results
Salivary 14-3-3 eta protein in RA patients (Mean ± SD; Median) (3.800 ± 2.659; 2.948) was significantly higher (P<0.001) as compared with that in healthy subjects (1.563 ± 0.620; 1.390). Serum 14-3-3 eta protein in RA patients (3.027 ± 1.244; 2.576) was significantly higher (P<0.001) compared with healthy subjects (1.757 ± 0.544; 1.73). RF, ACCP and CRP levels in patient with RA were highly significant (P<0.001) compared with healthy subjects as shown in (Table 2).
| Group I (n=15) |
Group II (n=69) |
p-value | |
|---|---|---|---|
| Salivary 14-3-3 eta protein (ng/mL) | 1.563 ± 0.620 (1.390) |
3.800 ± 2.659 (2.948) |
˂0.001 |
| Serum 14-3-3 eta protein (ng/mL) | 1.757 ± 0.544 (1.73) |
3.027 ± 1.244 (2.576) |
˂0.001 |
| Serum C-reactive protein (mg/L) | 3.284 ± 0.927 (2.978) |
5.614 ± 3.474 (4.831) |
˂0.001 |
| Serum Anti-cyclic citrullinated peptide (EU/mL) | 4.111 ± 1.310 (3.622) | 10.012 ± 4.714 (8.349) | ˂0.001 |
| Serum Rheumatoid factor (IU/mL) | 6.931 ± 1.945 (6.945) |
15.425 ± 9.845 (12.126) | ˂0.001 |
Table 2: Assessment of biomarkers in rheumatoid arthritis compared with health subjects.
In order to determine the sensitivity and specificity of salivary 14-3-3 eta protein. ROC analysis was performed comparing results between RA patients and healthy subjects. ROC curve showed that the (AUC) of 0.943 (95% confidence interval: 0.852-1.000) (P<0.001) as shown in Figure 1. At a cutoff of 1.8 ng/mL, salivary 14-3-3 eta protein had a sensitivity of 98.5% and specificity of 80%. The positive predictive value was 95%; negative predictive value was 85%.
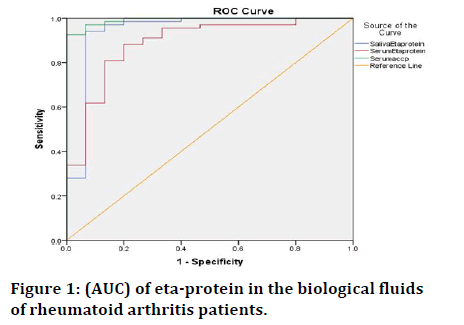
Figure 1: (AUC) of eta-protein in the biological fluids of rheumatoid arthritis patients.
ROC was performed to serum 14-3-3 eta protein comparing results from RA patients with healthy subjects. ROC curve showed that (AUC) was 0.888 (95% CI: 0.791-0.985; P<0.001) as shown in Figure 1. At a cutoff of 1.8 ng/mL, the ROC curve yielded a sensitivity of 95.6%, while the specificity of 66.7%. The Positive predictive value was 91.6%; negative predictive value was 75% (Table 3).
| Variable(s) | Area | p-value | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Serum eta-protein | 0.888 | 0 | 0.791 | 0.985 |
| Saliva eta-protein | 0.943 | 0 | 0.852 | 1 |
| Serum ACCP | 0.992 | 0 | 0.979 | 1 |
Table 3: The area, p-value and 95% Confidence Interval of the biological fluids levels of ETA-protein.
ROC was performed to serum ACCP comparing results from RA patients with healthy subjects. ROC curve showed that (AUC) was 0.992 (95% CI: 0.979-1.000; P<0.001) as shown in Figure 1. At a cutoff of 5.5 ng/mL, the ROC curve yielded a sensitivity of 98.5%, while the specificity of 80%. The Positive predictive value was 95.7%; negative predictive value was 92.3% (Table 4).
| Cutoff value (ng/mL) | Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
|---|---|---|---|---|---|
| Serum eta-protein | 1.8 | 95.6 | 66.7 | 91.6 | 75 |
| Saliva eta-protein | 1.8 | 98.5 | 80 | 95 | 85 |
| Serum ACCP | 5.5 | 98.5 | 80 | 95.7 | 92.3 |
Table 4: The levels of ETA-protein.
Table 5 displays the correlations of serum 14-3-3 eta protein concentration with clinical and serological variables in patients with RA. DAS28 (r=0.286, P=0.017) in RA patients. In addition, the correlation between ACCP and RF was significantly observed in patients with RA. However, correlation analyses of serum 14-3-3 eta protein concentration with ACCP (r=0.597, P˂0.001) and RF (r=0.518, P˂0.001) were significant. Serum levels of 14-3-3 eta protein were significant correlated with CRP levels (r=0.628, P˂0.001), in addition, correlation analyses of serum 14-3-3 eta protein with salivary 14-3-3 eta protein (r=0.302, P=0.012).
| Determinants | Serum | ||||
|---|---|---|---|---|---|
| DAS | ETA-protein | ACCP | Rheumatoid factor | C-reactive protein | |
| DAS | - | ||||
| Eta-protein | 0.286 (0.017) | - | |||
| ACCP | 0.261 (0.03) | 0.597 (<0.001) | - | ||
| Rheumatoid factor | 0.242 (0.045) | 0.518 (<0.001) | 0.463 (<0.001) | - | |
| C-reactive protein | 0.327 (0.006) | 0.628 (<0.001) | 0.572 (<0.001) | 0.335 (0.005) | - |
| Salivary eta protein | 0.302 (0.012) | - | |||
Table 5: Correlation coefficients (r) of 14-3-3 eta protein with clinical and serological measures in RA patients.
The results are expressed as mean ± SD (median). P-value was calculated using independent two sample t-test for continuous data
The results are expressed as percentages.
The results are expressed as Spearman rho correlation factor (P-value). ACCP: Anti-Cyclic Citrullinated Peptide.
Discussion
This study compared the levels of salivary 14-3-3 eta protein in RA patients with healthy individuals. However, the levels of serum 14-3-3 eta protein, RF, ACCP, and CRP in RA patients were compared with that of healthy individuals.
This study demonstrated that the levels of salivary 14-3-3 eta protein were highly significant in RA patients than in healthy individuals, and it was a good predictor for the risk of RA.
Serum 14-3-3 eta protein, RF, ACCP and CRP levels in RA patients were highly significant (P<0.001) in comparison with that in healthy subjects as shown in Table 2. Consistent with the previous research results [7].
However, this study was more comprehensive than previous studies. The current study did not only compare the levels of serum 14-3-3 eta protein between RA patients and healthy subjects, but also compared the levels of salivary 14-3-3 eta protein between RA patients and healthy subjects, and reveal to us that salivary 14-3-3 eta protein was able to use as a useful marker for RA.
A previous study [6] showed that the use of RF combined with serum 14-3-3 eta protein increased the detection rate from 57 to 75% compared to using RF alone for early-stage RA diagnosis and using ACCP in combination with serum 14-3-3 eta protein increased the detection rate from 59 to 72% compared to using ACCP alone. Another study showed that the use of 14-3-3 eta protein in the laboratory test along with either RF or ACCP will increase their diagnostic value [8,9]. Similar results were obtained in other studies, which indicate that serum 14-3-3 eta protein expression may be related to RF and ACCP levels in RA patients [10]. Guan, et al. Also found that the RA patient’s detection could be improved with the use of serum 14-3-3 eta protein. Zeng and Tan were reported that the diagnostic capture of RA patients was improved by combining serum protein 14-3-3 eta with rheumatoid factor and a citrullinated peptide antibody. In this study, the incremental benefit of adding the salivary 14-3-3 eta protein levels to serum 14-3-3 eta protein, CRP, ACCP and/or RF in the diagnosis of RF was demonstrated. The result of salivary 14-3-3 eta protein was correlated with serum 14-3-3 eta protein (r=0.302, P=0.012), and also correlations were found between serum 14-3-3 eta protein and DAS28 (r=0.286, P=0.017), ACCP (r=0.597, P˂0.001), RF (r=0.518, P˂0.001) and CRP (r=0.628, P˂0.001) (Table 5).
Maksymowych, et al. found that serum 14-3-3 eta protein significantly correlated with anti-citrullinated protein antibodies and rheumatoid factor in RA patients, but not with disease activity score in 28 joints or C-reactive protein. Another study from Japan [11] showed that serum 14-3-3 eta protein and DAS28, and CRP were correlated with significant results in RA patients. Correlation coefficients of serum 14-3-3 eta protein with DAS28 and CRP were (r=0.29, P<0.0001), and (r=0.20, P<0.05), respectively. Another study (2019), correlations were found between serum 14-3-3 eta protein and DAS28 (r=0.275), and CRP (r=0.250). Another study showed that serum 14-3-3 eta protein was positively correlated with DAS28, and increased in patients with RF positive and ACCP positive compared with that in patients with RF negative or ACCP negative [10]. On the basis of the above data, an inference was presented that serum 14-3-3 eta protein may reflect disease activity and inflammation to some extent, although these results were slightly different.
This is consistent because all of these biomarkers including 14-3-3 eta protein, RF and ACCP involved in the release of pro-inflammatory factors, including IL-1β and IL-6, which are associated with inflammation in RA [12]. Therefore, it was expected for salivary 14-3-3 eta protein levels to correlate with serum 14-3-3 eta protein levels in patients with RA in the present study.
In this study, the sensitivity of salivary 14-3-3 eta protein was 98.5%, while the specificity of salivary 14-3-3 eta protein was 80%, which represented higher than the results of serum 14-3-3 eta protein with the sensitivity of 95.6% and specificity of 66.7%.
Area under Curve (AUC) of salivary 14-3-3 eta protein was 0.943 (95% CI: 0.852-1.000; P<0.001), which appear higher compared to Area Under Curve (AUC) of serum 14-3-3 eta protein was 0.888 (95% CI: 0.791-0.985; P<0.001) as shown in (Figure 1) at the same cutoff of 1.8 ng/mL. The results indicated that adding salivary 14-3-3 eta protein to serum 14-3-3 eta protein could discriminate more patients with RA.
Recently, et al. Suggested that ACCP was the most valuable in the diagnosis of RA; their results showed that ACCP was with the highest specificity (94.5%). The Areas under the Curve (AUC) of ACCP was 0.89; which appeared comparable to our study results regarding ACCP, the Areas under the Curve (AUC) of ACCP was 0.992 indicating a high level of overall accuracy (13).
Maksymowych, et al. investigated the sensitivity/ specificity for the combination of 14-3-3 eta/ACCP (0.71/0.92) and RF/ACCP (0.71/0.84) in RA patients their study was performed on 234 patients (99 of them were in the early stages of the disease) and the results were compared with a control group (n=385). According to their study, when the sensitivity was the same, the specificity of the combination of 14-3-3 eta and ACCP was much greater than combination of the RF and ACCP [6].
Guan, et al. found that adding 14-3-3 eta to RF and ACCP testing increased diagnostic sensitivity for early RA patients [7].
In this study, ROC curve indicates the sensitivity of salivary 14-3-3 eta protein and serum ACCP have the same value 98.5% which is comparable with the sensitivity of serum 14-3-3 eta protein 95.6%, while the specificity of serum 14-3-3 eta protein was 66.7%, which represented lower than the specificity of both salivary 14-3-3 eta protein and ACCP which have the same value 80%.
To demonstrate excellent accuracy, the value of AUC should be 0.90-1 and AUC of 0.80-0.90 is considered to be good, [14]. Whereas in this study, salivary 14-3-3 eta protein AUC value was 0.943 which was higher than serum 14-3-3 eta protein 0.888 as which implied that adding salivary 14-3-3 eta protein to serum 14-3-3 eta could discriminate more patients with RA. While serum ACCP in this study was 0.992 showed more accuracy than 14-3-3 eta protein.
Salman, et al. found that 40 (88%) of their 45 patients who were seronegative for RF and ACCP were 14-3-3 eta positive and suggested that 14-3-3 eta protein is a valuable and promising marker in patients with seronegative RA. Other advantages of 14-3-3 eta protein as a RA marker are that adding 14-3-3 eta to RF or ACCP or the combination of all 3 markers would increase the diagnostic [15].
The strengths of this study were the paired saliva and serum samples. A limitation is that the small number of included patients.
Conclusion
Measurement of serum 14-3-3 eta protein and saliva 14-3-3 eta protein levels are significantly higher in rheumatoid arthritis. The addition of salivary 14-3-3 eta protein markers to serum 14-3-3 eta protein and ACCP might lead to better diagnosis and evaluation of RA. Salivary 14-3-3 eta protein offers a potential therapeutic role for further studies to confirm these promising results as a supplementary biomarker in RA.
References
- Zeng T, Tan L. 14-3-3 eta protein: a promising biomarker for rheumatoid arthritis. Biomarkers Med 2018; 12:917-925.
- Mohamed AH, Abdellatif A, El-Noshokaty EH. Serum level of 14-3-3eta (eta) protein as a diagnostic marker for rheumatoid arthritis and potential correlation with disease activity. MOJ Orthop Rheumatol 2017; 7:00279.
[Crossref]
- Yarlagadda LD, Jacob R, Rajasekhar DL, et al. Evaluation of a new biomarker 14-3-3 eta protein in diagnosis of rheumatoid arthritis. Indian J Rheumatol 2020; 15:175-180.
- PLCM van Riel L, Renskers. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin Exp Rheumatol 2016; 34:S40-S44.
- Smolen JS, Aletaha D, Mcinnes IB. An extensive literature review on rheumatoid arthritis. Rheumatoid arthritis. Lancet 2016; 388:2023–2038.
- Maksymowych WP, van der Heijde D, Allaart CF, et al. 14-3-3eta is a novel mediator associated with the pathogenesis of rheumatoid arthritis and joint damage. Arthritis Res Ther 2014; 16:1-11.
- Guan SZ, Yang YQ, Bai X, et al. Serum 14-3-3eta could improve the diagnostic rate of rheumatoid arthritis and correlates to disease activity. Ann Clin Lab Sci 2019; 49:57–62.
- Darwish NF, Hablas SA, Baiomy NN, et al. Evaluation of serum 14-3-3eta protein and Sema 3A levels in rheumatoid arthritis: diagnostic and prognostic value. Egypt Rheumatol Rehabil 2020; 47:43.
- Van Beers-Tas MH, Marotta A, Boers M, et al. A prospective cohort study of 14-3-3eta in ACCP and/or RF-positive patients with arthralgia. Arthritis Res Ther 2016; 18:76.
- Jianxin Tu, Chen X, Dai M, et al. Serum levels of 14 3 3eta are associated with increased disease risk, activity and duration of rheumatoid arthritis in Chinese patients. Exp Ther Med 2020; 20:754-761.
- Hirata S, Marotta A, Gui Y, et al. Serum 14-3-3eta level is associated with severity and clinical outcomes of rheumatoid arthritis, and its pre-treatment level is predictive of DAS28 remission with tocilizumab. Arthritis Res Ther 2015; 17:280.
- Zhang C. Flare up of cytokines in rheumatoid arthritis and their role in triggering depression: Shared common function and their possible applications in treatment (Review). Biomed Rep 2020; 14.
- HU T, Liu Y, Tan L, et al. Value of serum collagen triple helix repeat containing-1 (CTHRC1) and 14-3-3η protein compared to anti-CCP antibodies and anti-MCV antibodies in the diagnosis of rheumatoid arthritis. British J Biomed Sci 2021; 78.2:67-71.
- Nindrea, Ricvan Dana, et al. Diagnostic accuracy of different machine learning algorithms for breast cancer risk calculation: a meta-analysis. Asian Pac J Cancer Prev 2018; 19.7:1747.
- Salman E, Cetiner S, Boral B, et al. Importance of 14‐3‐3eta, anti‐CarP, and anti‐Sa in the diagnosis of seronegative rheumatoid arthritis. Turk J Med Sci 2019; 49:1498‐1502.
Author Info
Ayoob Jasim Mohammed1*, Ameena Ryhan Diajil1 and Fedan Ihsan Hassan2
1Department of Oral Medicine, College of Dentistry, University of Baghdad, Baghdad, Iraq2Department of Radiologist Specialist, Baghdad Medical City Complex, Baghdad Teaching Hospital, Baghdad, Iraq
Citation: Ayoob Jasim Mohammed, Ameena Ryhan Diajil, Fedan Ihsan Hassan, Assessment of Serum and Salivary 14-3-3 ETA Protein in Rheumatoid Arthritis Patients, J Res Med Dent Sci, 2022, 10 (10): 007-012.
Received: 19-Aug-2022, Manuscript No. JRMDS-22-51582; , Pre QC No. JRMDS-22-51582(PQ); Editor assigned: 22-Aug-2022, Pre QC No. JRMDS-22-51582(PQ); Reviewed: 04-Sep-2022, QC No. JRMDS-22-51582; Revised: 21-Oct-2022, Manuscript No. JRMDS-22-51582(R); Published: 28-Oct-2022
