Research - (2022) Volume 10, Issue 2
Assessment of Surface Roughness of Teflon Archwires Immersed in Mouthwashes (An In-vitro Comparative Study)
Mena Hasan Abdulqader* and Sami Kadhum Al-joubori
*Correspondence: Mena Hasan Abdulqader, Department of Orthodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Aim: the goal of this research is to see how two different mouthwashes affect the surface roughness of Teflon orthodontic arch wires. Materials and methods: round (0.018 inch) Teflon esthetic nickel titanium orthodontic arch wires were immersed in either Sidra Zac (fluoridated mouthwash), Biofresh (non-fluoridated mouth wash), or distilled water (control) for 1 week, 3weeks and 6 weeks at 37°C. After the immersion process, the surface roughness of arch wires was measured using atomic force microscopy. A one-way analysis of variance and Tukey's post hoc α=0.05, were used to analyze the roughness testing data. Results: ANOVA test has been shown a non-significant difference among immersion media after 1week period while after 3 weeks and 6 weeks there was a highly significant difference between different media. Conclusion: the distilled water didn’t increase the surface roughness significantly even after 6 weeks immersion period. The non-fluoridated mouthwash increases the surface roughness significantly after 6 weeks while the fluoridated mouthwash increases the roughness significantly after 3 weeks only with higher values. So that the fluoridated mouthwash is considered more aggressive on Teflon esthetic arch wires.
Keywords
Teflon arch wires, Mouthwashes, Surface roughness, Atomic force microscopy
Introduction
A high percentage of adult and adolescent patients reject labial brackets and wires increasingly. As a result, more attractive orthodontic appliances, for example brackets that are fixed on lingual surface or translucent plastic aligners, have been developed. However, there are several disadvantages to these devices in terms of cost, convenience of use, and efficacy [1,2]. The invention of ceramic brackets has enhanced the appearance and acceptance of labial fixed appliances dramatically [3]. There have also been attempts to make esthetic orthodontic arch wires by coating them with polymer or Teflon [4]. Teflon is a carbon-fluorine based synthetic polymer. This arch wire is nonreactive, heat tolerant, and hydrophobic because of the carbon-fluorine bonds' strength [5]. Teflon's coating not only protects the wire from corrosion, but it also improves wire aesthetics and decreases friction. It has the third lowest coefficient of friction of any solid ever known and a Coating thickness of 0.002. These wires are available in natural tooth color and also in blue, green or purple colors [6].
When compared to ordinary stainless steel and nickeltitanium (NiTi) arch wires, these arch wires have poor esthetic value since the coating is non-durable and deteriorates rapidly in the intraoral environment, resulting in increased surface roughness [7]. The Teflon coating is applied by thermal spraying to an orthodontic wire, a method in which finely heated materials are sprayed to a surface to form a coating in a molten state [8]. The surface roughness of orthodontic arch wires is an essential component in determining the effectiveness of arch wire-guided tooth movement. A significant factor in evaluating the efficacy of arch wire-guided tooth movement is the surface roughness of orthodontic arch wires. The surface quality of arch wires influences the area of surface contact, corrosion behavior, and biocompatibility, as well as color stability and appliance performance when using sliding mechanics [9]. In addition, A surface roughness (SR) investigation discovered that after oral exposure, both the peeled and remaining coated areas had a higher SR [10]. By increasing the SR, the coefficient of friction, which is a necessary factor in deciding the effectiveness of sliding tooth movement can be increased [9]. Furthermore, rough surfaces establish new plaque retention sites, resulting in mechanical plaque removal being compromised [11]. Plaque build-up, decay, decalcification, and other problems can be exacerbated by the placement of orthodontic bands and brackets. As a result, in order to avoid developing dental pathosis, orthodontic patients must practice thorough plaque control [12]. So that, mouthwashes in addition to mechanical tooth cleaning are recommended [13]. However, their components may cause stainless steel and titanium alloys to corrode and discolor. The creation of a passive oxide layer protects stainless steel and titanium wires against corrosion. The arch wire may be corroded if this layer deteriorates which lead to increasing in surface roughness [14]. So that this study has been established to evaluate the effect of mouthwashes on surface roughness of esthetic arch wires.
Materials and Methods
One type of commercially available round 0.018inch esthetic nickel titanium orthodontic arch wire is investigated in this study. This arch wire is Teflon nickel titanium esthetic orthodontic arch wires from [DTC company, Chinese].
Two different mouthwash solutions are used which involved Sidra Zac mouthwash (0.12% chlorhexidine digluconate, 0.05 fluoride, PH=6, Alpha pharma, Turkey] and Biofresh mouthwash (0.12% chlorhexidine digluconate without fluoride, PH=5.3, Scitra Co, U.A.E]. Distilled water used as control media.
The samples (72 pieces as a whole). 8 pieces from this type of arch wire will be chosen to evaluate surface roughness as received. Each time 24 pieces from Teflon arch wire will be picked for immersion in different solutions. These 24 pieces divided into groups of 8 pieces to be put in distilled water for 1 min for different periods of time 1week, 3weeks, 6weeks, and then do the same as for the second group of 24 pieces to conclude what will happen when be put in Sidra Zac mouthwash. Finally, the last group of 24 pieces was immersed in Biofresh mouthwash repeating the same process and then assessing the surface roughness of each piece of wires with atomic force microscopy (Figure 1).
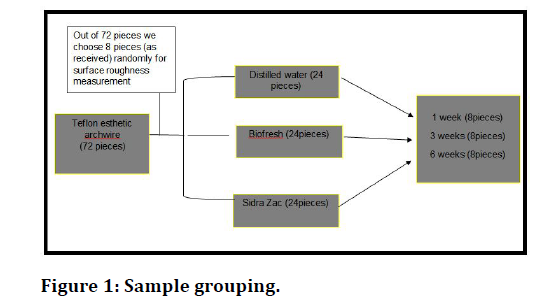
Figure 1. Sample grouping.
Specimen preparation
During this step, the arch wires were removed from their packaging and 20 mm segments with digital vernier were measured and marked by a permanent marker. Finally, these segments were cut from both straight sections of the preformed arch wires by arch wire cutter. To remove the contaminated layer that formed during storage, the samples were sterilized by washing sequentially with distilled water, ethanol, distilled water and finally dried with a filter paper.
Samples immersed in solutions are prepared in the following manner
To begin, equal amounts of epoxy steel adhesive squeezers have been put on cement slab and mix them together. The tweezer was utilized to handle each sample in a non-contaminating manner, then small amount of epoxy adhesive was added to the end of wire to form a tiny ball which help in tying the dental floss. The artery forceps were used to hold each sample from the end of the piece, then the dental floss was utilized to make a knot around the teeny ball.
Each sample was placed in a separate glass container in specific way to prevent touching the container's wall. For 1 minute per day, the solution was added to the container such that immersing the sample completely and excluding the epoxy ball. During immersion, the samples were incubated at 37 degrees Celsius, which is the temperature of oral cavity, and then rinsed with distilled water. After that the samples incubated in distilled water at the same temperature. After 1 week, 3 weeks, and 6 weeks, surface roughness measures were recorded. After the intervals had passed, the arch wires were removed, washed with distilled water, dried with dry air, and placed in petri dishes for examination.
How to prepare the specimen for testing?
AFM have been used in this study which needs a microscopic slide. The vernier was used to measure the small segments of (2*2) cm which were obtained by slicing the microscopic slides via a special cutting tool called a diamond cutting pen.
Each wire sample was put on a small segment of microscopic slides and glued with epoxy steel adhesive after mixing. After that, a petri dishes were used to hold the wire samples and secure them in the same position with adhesive tape such that it can’t move in any direction. Sticky labels were utilized to group the samples according to the immersion period and solution type.
The AFM utilized to analyze surface roughness was the NTEGRA prima NT-MDT, which has a silicon probe mounted on a cantilever. It was programmed to scan for 540 seconds in a tapping mode (9 minutes). The scan parameters were controlled using the NOVA. SPM software, and the images were processed using the image analysis P9 program. It was a room-temperature scan. The area that has been examined was the middle of arch wires. size of scanned area was 30 X 30μm² with a resolution of 256×256 pixels and 0.8line/s scan speed. On the monitor of the computer attached to the AFM, a 3D view of the surface of arch wire was displayed.
Results
The data was statistically analyzed using SPSS Statistics software version 25.0 ((IBM Company, New York, USA). The Shapiro-Wilk test was used to determine the normality of the data distribution, which showed the data were normally distributed. The ANOVA test was used to analyze statistical differences. The level of significance was set at P=0.05.
Parametric tests had been used as follows:
Inferential statistics
Comparison between the immersion media
As showed in Table 2 and Figure 2, ANOVA test was used. The test showed a highly significant difference between different immersion media after 3 weeks and 6 weeks, therefore, 1 week immersion period not enough to cause a statistical difference between groups. The smallest values of surface roughness were found during immersion in distilled water in comparison to as received.
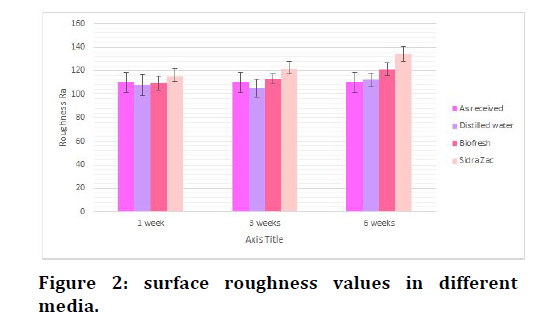
Figure 2. surface roughness values in different media.
| Intervals | Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|---|
| 1 week | Between Groups | 250.835 | 3 | 83.612 | 1.63 | 0.205 |
| Within Groups | 1436.202 | 28 | 51.293 | |||
| 3 weeks | Between Groups | 1119.886 | 3 | 373.295 | 8.923 | 0 |
| Within Groups | 1171.354 | 28 | 41.834 | |||
| 6 weeks | Between Groups | 2857.211 | 3 | 952.404 | 21.116 | 0 |
| Within Groups | 1262.918 | 28 | 45.104 | |||
Table2: ANOVA test for the comparison between different media.
Descriptive statistics
The results of mean values, standard deviations (SD), maximum (Max.) and minimum (Min.) of surface roughness of each group presented in Table 1. The surface roughness values of tested samples expressed in nanometer (nm).
| media | intervals | No. | Mean | SD | Min. | Max. |
|---|---|---|---|---|---|---|
| As received | 8 | 110.22 | 8.52 | 96.72 | 119.98 | |
| Distilled water | 1 week | 8 | 107.7 | 8.95 | 92.93 | 117.64 |
| 3 weeks | 8 | 105.3 | 7.58 | 94.33 | 114.57 | |
| 6 weeks | 8 | 112.07 | 6.15 | 101.87 | 118.88 | |
| Biofresh | 1 week | 8 | 109.52 | 5.86 | 102.3 | 117.11 |
| 3 weeks | 8 | 113.21 | 4.19 | 108.01 | 120.7 | |
| 6 weeks | 8 | 121.02 | 5.22 | 112.33 | 126.89 | |
| Sidra Zac | 1 week | 8 | 115.25 | 4.23 | 110.06 | 121.01 |
| 3 weeks | 8 | 121.58 | 4.42 | 116.24 | 127.46 | |
| 6 weeks | 8 | 134.11 | 6.52 | 125.04 | 142.3 |
Table 1: Descriptive statistics of surface roughness of different groups.
group while the highest values of surface roughness were found during immersion in fluoridated mouthwashes (Sidra Zac) means that it was the most aggressive agent on esthetic arch wires. The Post Hock Tukey’s test Table 3 has shown the statistical difference was between as received-Sidra Zac and between distilled water-Sidra Zac after 3week immersion period. In 6 weeks immersion period the significant difference was between as received- Biofresh, as received -Sidra Zac, distilled water- Sidra Zac and Biofresh- Sidra Zac.
B-Comparison between immersion period
ANOVA test was used Table 4 and Figure 3 which illustrated that the surface roughness doesn’t change significantly in distilled water with time, as the distilled water is not an aggressive media in contrast to Biofresh in which the surface roughness increases significantly after 6 weeks. While in Sidra Zac mouthwashes, the surface roughness increases significantly after 3 weeks as the roughness values increases by the time. Post Hock Tukey’s test Table 5 illustrated the significant difference was between 1 week- 6 weeks and between 3 weeks- 6 weeks in both types of mouthwashes.
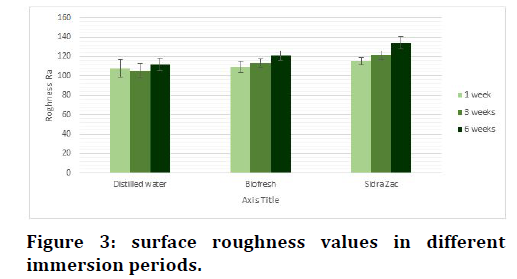
Figure 3. surface roughness values in different immersion periods.
| Media | 1 week | 3 weeks | 6 weeks | ||||
|---|---|---|---|---|---|---|---|
| Mean difference | P-value | Mean difference | P-value | Mean difference | P-value | ||
| As received | Distilled water | 4.91` | 0.43 | 1.84 | 0.94 | ||
| Biofresh | 2.99 | 0.79 | 10.8 | 0.01 | |||
| Sidra Zac | 11.36 | 0.008 | 23.89 | 0 | |||
| Distilled water | Biofresh | 7.91 | 0.09 | 8.95 | 0.057 | ||
| Sidra Zac | 16.28 | 0 | 22.04 | 0 | |||
| Biofresh | Sidra Zac | 8.36 | 0.06 | 13.08 | 0.003 | ||
Table 3: The Post-hoc Tukeyâ??s test after ANOVA test.
| Media | Sum of Squares | df | Mean square | F-test | Sig. | |
|---|---|---|---|---|---|---|
| Distilled water | Between Groups | 188.209 | 2 | 94.105 | 1.608 | 0.224 |
| Within Groups | 1229.355 | 21 | 58.541 | |||
| Biofresh | Between Groups | 552.038 | 2 | 276.019 | 10.453 | 0.001 |
| Within Groups | 554.525 | 21 | 26.406 | |||
| Sidra Zac | Between Groups | 1473.716 | 2 | 736.858 | 27.612 | 0 |
| Within Groups | 560.41 | 21 | 26.686 | |||
Table 4: ANOVA test for the comparison between immersion periods.
| Media | Intervals | Mean difference | P-value | |
|---|---|---|---|---|
| Distilled water | 1 week | 3 weeks | ||
| 6 weeks | ||||
| 3 weeks | 6 weeks | |||
| Biofresh | 1 week | 3 weeks | 3.69 | 0.34 |
| 6 weeks | 11.5 | 0.001 | ||
| 3 weeks | 6 weeks | 7.81 | 0.016 | |
| Sidra Zac | 1 week | 3 weeks | 6.32 | 0.058 |
| 6 weeks | 18.85 | 0 | ||
| 3 weeks | 6 weeks | 12.53 | 0 | |
Table 5: The Post-hoc Tukeyâ??s test after ANOVA test.
Discussion
Compromised oral hygiene one of the major drawbacks of orthodontic treatment, this results in demineralization of the enamel, white spots, and caries. Periodontal problems may develop in some patients while undergoing orthodontic treatment, necessitating the use of different mouthwashes to reduce plaque accumulation [15].
Roughness refers to the surface's texture and how it interacts with its surroundings. It is distinguished by its amplitude (vertical), spacing (horizontal), and hybrid parameters. Materials, coatings, manufacturers and manufacturing techniques have all shown to influence archwire surface structure [16]. The most significant effect is that of surface roughness. Plaque accumulation is accelerated by increasing surface roughness and free surface energy. In the leveling stage, lower friction improves the sliding movement between the wire and the bracket resulting in faster tooth movement, less wasted force, and better anchorage control [17].
Surface profilometry was the primary method for investigating surface roughness, a technique in which a tiny tip was used to scan the topography along a single line in a predetermined area. The main disadvantage of this technology is that it is an invasive method, as well as, surface defects next to the scan line cannot be measured. As a result of the growing demand for nondestructive and noninvasive procedures, novel analysis approaches had developed atomic force microscopy (AFM) based on an optical method.
In this research, the surface roughness of orthodontic wires was assessed qualitatively using the AFM technique. The AFM is a type of scanning probe microscope that collects information on detected surfaces by using interatomic interactions. AFM uses a sensor, which is a sharp point that interacts with the specimen surface to create image [18]. This method is non-invasive and only necessitates a small amount of sample preparation. Furthermore, the AFM technique produces simultaneous 2-D and 3-D images, allowing specimens to be re-evaluated without damage [17].
This study investigated the effect of fluoridated and nonfluoridated mouthwashes on surface roughness of Teflon esthetic archwires in comparison to distilled water which used as control media. It is found that the surface roughness of Teflon archwires has been increased with time in both types of mouthwashes in comparison to distilled water. The increased roughness in fluoridated mouthwashes is most likely the result of topical fluoride's corrosive effect on titanium-based orthodontic archwires. Because of titanium's high affinity for hydrogen, degradation and loss of the oxide film on the surface, the underlying alloy will be exposed, resulting in corrosion and absorption of hydrogen ions from the aqueous-based solution. The diffusion of hydrogen through interstitial sites, dislocations, and grain boundaries reacting with lattice atoms to form hydride phases, particularly titanium hydride, has been proposed to explain hydrogen absorption and associated embrittlement of titanium-based alloys. Titanium hydrides have been observed to establish a bodycentered tetragonal structure, which is thought to be the source of the alloy's surface property degradation [15,19]. The present study agrees with [20] who stated the fluoridated mouthwash change the surface morphology of coated arch wires. Also, in agreement with [14] who concluded both the neutral and the acidulated phosphate fluoride agents cause corrosive changes in surface topography.
In Biofresh mouthwashes the increased roughness may attributed to low PH and increased acidity which cause the destruction of protective layer, this resulted in increased hydrogen peroxide penetration. In addition, Hydrogen peroxide can cause the release of large amounts of OH radicals in metal surfaces which has the potential to cause Ni-Ti wire surface layers to be damaged [21]. This study in agreement with [22] who reported that soft drinks with a low PH level have been found to corrode the surface of Ni-Ti orthodontic arch wires in different ways depending on the surface pattern.
The increased surface roughness cannot be attributed to Chlorohexidine component, as chlorhexidine-containing mouth rinses may be prescribed as non-destructive prophylactic agents [23]. Due to the presence of fluoride ion with increased acidity in Sidra Zac mouthwash, the surface roughness was higher in comparison to Biofresh mouthwash which has low PH, because the effect of Fluoride and PH together is stronger than the effect of low PH only on surface roughness [24]. In our study, the maximum exposure time was 6 weeks. The exposure time may differ in clinical situations. Mastication and the oral environment may also affect the layer of orthodontic wires. However, this effect was not assessed in this in vitro test. We used one type of orthodontic arch wires that were clinically available. Despite their comparable composition, wires from different manufacturers may have varied surface roughness.
Limitations of the Study
As it is an in-vitro study it is difficult to simulate the actual oral conditions such as oral hygiene habits, oral pH, biological variables, the duration of intraoral exposure, and the physical and chemical properties of ingested food and liquid. This is in contrast to the clinical circumstances when the wire was exposed to chemical solutions while being ligated into brackets on misaligned teeth. In this situation, the arch wires developed a significantly increased rate of surface roughness in comparison to non-deflected arch wires.
Conclusion
The surface roughness of Teflon esthetic Ni-Ti orthodontic arch wires was investigated using 1 week, 3 weeks and 6 weeks immersion periods in two types of mouthwashes which included fluoridated and nonfluoridated mouthwashes. The immersion media and immersion time had a statistically significant influence on surface roughness variation. In sidra Zac mouthwash, the significant increase in surface roughness values obtained after 3 weeks immersion period. In Biofresh mouthwash, the significant increase in surface roughness obtained after 6 weeks immersion period. According to this study the Teflon arch wires can be left inside the patient mouth who takes the non- fluoridated mouthwash for longer period than the patient who uses a fluoridated mouthwash. Thus, when evaluating the effectiveness of arch-guided tooth movement, the increase in surface roughness of Ni-Ti orthodontic arch wires in commercial fluoride-containing environments and acidulated mouthwashes should be considered.
Funding
Self-funded.
Conflict of Interest
None.
Acknowledgements
Authors are grateful to ministry of science and technology for accomplishing the measurement task.
References
- Ata-Ali F, Ata-Ali J, Ferrer-Molina M, et al. Adverse effects of lingual and buccal orthodontic techniques: A systematic review and meta-analysis. Am J Orthod Dentofac Orthop 2016; 149:820-829.
- Rossini G, Parrini S, Castroflorio T, et al. Efficacy of clear aligners in controlling orthodontic tooth movement: a systematic review. Angle Orthod 2015; 85:881-889.
- Rosvall MD, Fields HW, Ziuchkovski J, et al. Attractiveness, acceptability, and value of orthodontic appliances. Am J Orthod Dentofac Orthop 2009; 135:276-e1.
- Bradley TG, Berzins DW, Valeri N, et al.An investigation into the mechanical and aesthetic properties of new generation coated nickel-titanium wires in the as-received state and after clinical use. Eur J Orthod 2014; 36:290-296.
- Farronato G, Maijer R, Carìa MP, et al.The effect of Teflon coating on the resistance to sliding of orthodontic archwires. Eur J Orthod 2012; 34:410-417.
- Philip N, Sunny S, George LA, et al. Newer orthodontic archwires: Imparting efficacy to esthetics. Int J Oral Health Dent 2016; 2:102-105.
- Nathani R, Daigavane P, Shrivastav S, et al. Esthetic arch wires-A review. Int J Adv Res 2015; 3:743-751.
- Arango S, Peláez-Vargas A, GarcÃa C. Coating and surface treatments on orthodontic metallic materials. Coatings 2013; 3:1-5.
- Bourauel C, Fries T, Drescher D, et al. Surface roughness of orthodontic wires via atomic force microscope, laser specular reflectance, and profilometry. Eur J Orthod 1998; 20:79-92.
- Da Silva DL, Mattos CT, De Araújo MV, et al. Color stability and fluorescence of different orthodontic esthetic archwires. Angle Orthod 2013; 83:127-32.
- Steinberg D, Eyal S.Initial biofilm formation of Streptococcus sobrinus on various orthodontics appliances. J Oral Rehab 2004; 31:1041-1045.
- Schrems HT.Patient motivation for oral hygiene from the dentist's viewpoint. Deutsche Zahnarztliche Zeitschrift 1979; 34:445-448.
- Kahlon S, Rinchuse D, Robison JM, et al. In-vitro evaluation of frictional resistance with 5 ligation methods and Gianelly-type working wires. Am J Orthod Dentofac Orthop 2010; 138:67-71.
- Walker MP, Ries D, Kula K, et al.Mechanical properties and surface characterization of beta titanium and stainless steel orthodontic wire following topical fluoride treatment. Angle Orthod 2007; 77:342-348.
- Hammad SM, Al-Wakeel EE, Gad ES. Mechanical properties and surface characterization of translucent composite wire following topical fluoride treatment. Angle Orthod 2012; 82:8-13.
- Rudge P, Sherriff M, Bister D.A comparison of roughness parameters and friction coefficients of aesthetic archwires. Eur J Orthod 2015; 37:49-55.
- Mousavi SM, Shamohammadi M, Rastegaar Z, et al. Effect of esthetic coating on surface roughness of orthodontic archwires. Int Orthod 2017; 15:312-21.
- Ryu SH, Lim BS, Kwak EJ, et al.Surface ultrastructure and mechanical properties of three different white-coated NiTi archwires. Scanning 2015; 37:414-21.
- Huang HH. Variation in surface topography of different NiTi orthodontic archwires in various commercial fluoride-containing environments. Dent Material 2007; 23:24-33.
- Aghili H, Yassaei S, Eslami F. Evaluation of the effect of three mouthwashes on the mechanical properties and surface morphology of several orthodontic wires: An in vitro study. Dent Res J 2017; 14:252.
- Omidkhoda M, Poosti M, Sahebnasagh Z, et al. Effects of three different mouthwashes on the surface characteristics of nickel-titanium and stainless steel archwires in orthodontics. J Dent Material Tech 2017; 6:19-26.
- Abalos C, Paul A, Mendoza A, et al. Influence of soft drinks with low pH on different Ni-Ti orthodontic archwire surface patterns. J Material Eng Performance 2013; 22:759-766.
- Nik TH, Hooshmand T, Farazdaghi H, et al.Effect of chlorhexidine-containing prophylactic agent on the surface characterization and frictional resistance between orthodontic brackets and archwires: An in vitro study. Progress Orthod 2013; 14:1-8.
- Perinetti G, Contardo L, Ceschi M, et al. Surface corrosion and fracture resistance of two nickel-titanium-based archwires induced by fluoride, pH, and thermocycling. An in vitro comparative study. Eur J Orthod 2012; 34:1-9.
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Author Info
Mena Hasan Abdulqader* and Sami Kadhum Al-joubori
Department of Orthodontics, College of Dentistry, University of Baghdad, IraqDepartment of Orthodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq
Received: 29-Jan-2022, Manuscript No. JRMDS-22-54390; , Pre QC No. JRMDS-22-54390 (PQ); Editor assigned: 31-Jan-2022, Pre QC No. JRMDS-22-54390 (PQ); Reviewed: 14-Feb-2022, QC No. JRMDS-22-54390; Revised: 18-Feb-2022, Manuscript No. JRMDS-22-54390 (R); Published: 25-Feb-2022
