Research - (2022) Volume 10, Issue 2
Association of Anterior Implant Placement and Advancement of Flap
Santhosh Bala S1, Abhinav RP1*, Subhasree R2 and Thiyaneswaran N2
*Correspondence: Abhinav RP, Department of Oral and Maxillofacial Surgery, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, India, Email:
Abstract
Introduction: Implant osseointegration is defined as direct contact between the implant surface and the bone as measured under a light microscope. When enough bone volume surrounds the full surface of the implant, it has a better chance of surviving. Bone grafting may be required to ensure enough osseous support. This can happen at a variety of times, including after extraction for socket augmentation and in a staged or simultaneous approach with implant placement for ridge augmentation. Flap reflection is required for access to determine bone morphology, quantity, quality, and key anatomical features during bone grafting procedures. The flap characteristics and the outcomes of regenerative operations may be influenced by a variety of incision patterns and reflection approaches. The aim of this study is to determine the association of anterior implants and flap advancement. Materials and methods: In this retrospective study, the data was collected from the hospital database and further analysis was done and the results were tabulated. A statistical analysis of the collected data regarding the necessity of flap advancement during anterior implant placement Results: The sample size was found to be 2,116. 16.19% were in the age group 18-30 years, 39.88% were in the age group 30-50 years, 40.39% were in the age 50-70 years and 3.56% were in the age group 70-90 years. 67.08% of the cases were males and 32.92% were females. 28.29% of the cases needed flap advancement, while 71.71% of the cases did not require flap advancement. 61.74% of the cases were maxillary anterior and 38.26% of the cases were mandibular anterior. Discussion: In the current study, it can be concluded that there is no significant association between anterior implant placements. It is not dependent on the site of the implant. Previous study conducted by Zazou et al. concluded that flap advancement can be used in mandibular posteriors, especially if ridge augmentation is needed. In cases of anterior implant placements, Flap advancement is usually not needed. But a true association cannot be made between the implant site and necessity for flap advancement, as flap advancement is decided based on various factors mentioned above. Conclusion: Thus, it can be concluded that there is no association between anterior implant placement and flap advancement. Flap advancement is decided based on various factors, but the implant site alone is not determinative of the need for advancement of flap.
Keywords
Anterior implants, Flap advancement, Osseointegration, Innovative treatment
Introduction
Implant osseointegration is defined as direct contact between the implant surface and the bone as measured under a light microscope. When enough bone volume surrounds the full surface of the implant, it has a better chance of surviving. Bone grafting may be required to ensure enough osseous support. This can happen at a variety of times, including after extraction for socket augmentation and in a staged or simultaneous approach with implant placement for ridge augmentation. Flap reflection is required for access to determine bone morphology, quantity, quality, and key anatomical features during bone grafting procedures. The flap characteristics and the outcomes of regenerative operations may be influenced by a variety of incision patterns and reflection approaches [1].
Flap designs based on the need for flap advancement are categorized into 3 types- Mild flap advancement, moderate flap advancement and major flap advancement. Mild flap advancement is employed when the flap should be advanced 1-3 mm. When a buccal plate defect likely to result in implant fenestration is present, Aesthetic buccal flap approach was advocated for immediate implantation in the aesthetic zone. This flap is very useful when existing alveolar bone loss, thin tissue biotype, or a combination of the two causes flap reflection recession, papilla height loss, and unsatisfactory aesthetics. It involves creating a full-thickness flap with a horizontal incision 3 mm apical to the free gingival margin in keratinized tissue, followed by two vertical releasing incisions extending past the MGJ. Before or after the atraumatic evacuation of the hopeless tooth, the flap might be performed [2]. The flap is replaced, and the vertical and horizontal incisions are sutured together to reduce stress. The implant should be temporised as soon as possible to maximise soft tissue support [3]. Mucogingival pouch flap is used when aesthetic considerations are low. This procedure is also used to graft a buccal dehiscence or fenestration defect after implant placement. Additional flap extension is achieved by periosteal scoring incisions (for additional 2–3 mm advancement) after flap reflection and grafting with the SBA technique and a collagen membrane [4]. Periosteal pocket flap is suitable for staged GBR before implant insertion and is proposed for horizontal augmentation of knife-edge ridges. The periosteal pocket promotes graft containment as well as stability. The coronal section of the graft is covered with a collagen membrane. Horizontal mattress suturing is done in two steps through the lingual flap, each time engaging the buccal periosteal and mucosal layers independently [5]. Lateral incision technique provides targeted horizontal ridge augmentation. When compared to crestal incisions, the combined flap design is said to reduce soft tissue issues. For space preservation, tenting screws can be used with a bone graft and a nonresorbable, fixed ePTFE membrane. For tension-free primary closure, a horizontal mattress and simple interrupted sutures are employed [6].
Moderate flap advancement techniques are used for when advancement needed is 4-6 mm. Buccal pedicle flap/graft design is utilised for socket preservation or rapid implant insertion to allow for soft tissue covering while avoiding a disparity in the MGJ, vestibule loss, and limited keratinized tissue, all of which can occur if the flap is overextended. Instead, a pedicle or free gingival graft method uses buccal keratinized gingiva from a neighbouring tooth as the donor for soft tissue covering over the membrane. Any graft that is left over can be used to restore the donor location [7]. Vascularised periosteal membrane is used to perform primary closure over grafted sockets. It is possible to utilise an absorbable membrane or not. The additional flap extension is performed by internally splitting the palatal flap in half from an apical aspect to produce a pedicle and unfolding it over the graft site, resulting in full-thickness buccal and palatal flaps. The buccal flap is restored over the expanded tissue and single interrupted sutures are used to close the wound [8]. Lingual pedicle uses a rotational split-thickness connective tissue pedicle from the palate to boost flap length for primary soft tissue closure over grafted extraction sites or insufficient ridges, similar to the vascularized periosteal membrane. At the most apical extent of the vertical releasing incisions, the buccal flap design comprises horizontal incisions of 3 to 4 mm up to 6 to 10 mm in length. In a case series, this flap maintained soft tissue covering for 6 months in 98.8% of the 173 sites [9]. Coronally positioned palatal sliding flap is used to enlarge the zone of keratinized tissue and offer primary coverage of barrier membranes during implant implantation and simultaneous grafting, split-thickness planes of palatal tissue are created from a full-thickness flap to allow for sliding and rotation similar to a garage door (10). Like a free gingival graft donor site, the underlying de-epithelialized palatal tissue will reepithelialize [10,11].
Major flap advancement is employed when the advancement needed is 7mm or greater. Hockey stick flap was proposed by Tinti and Parma-Benfenati to accommodate extensive vertical ridge augmentation around implants. The vertical incisions of the fullthickness buccal flap have "hockey stick"–shaped apical extensions. These incisions are often known as "cutback" incisions [12]. The lingual flap reflection requires the mylohyoid muscle being raised while important structures in the mouth's floor are contained and protected [13]. A coronally positioned palatal flap is employed in the maxilla. The periosteum (as well as muscle fibres in the mandible) is separated from the flaps' outer, mucosal component. Horizontal mattress “Ustitches'' spaced 3 mm apart from the “first line of closure,” which is followed by simple interrupted sutures [14]. Remote flap is used for horizontal or vertical ridge augmentation. This flap is a version of Tinti and Parma- Benfenati's "Hockey stick" flap design. Extending the flap 5 mm beyond the graft site in edentulous areas is one modification (compared with 3 mm). Sutures are put in place using a horizontal mattress and simple interrupted sutures [15]. Double flap was created for GBR in the posterior jaw, both vertically and horizontally. The design of a mid crestal horizontal incision with a single vertical incision at the mesial aspect of the flap was based on anatomical considerations in order to protect blood flow to the avascular crestal section of the edentulous ridge while avoiding essential structures. The mucosal and periosteal flaps are lifted and sutured independently, just as the periosteal pocket flap [16]. To generate a twofold partial thickness buccal flap and a coronal positioned palatal sliding flap, Multilayered approach employs plastic and microsurgery concepts [17].
Our team has extensive knowledge and research experience that has translated into high quality publications [18-37]. The aim of this study is to determine the association of anterior implants and flap advancement.
Material and Methods
This retrospective study examined the records of patients from 01 June 2019 to 31st March 2021 who visited Saveetha Dental College and Hospitals. Ethical approval was taken from the institutional review board. The study population included patients who underwent implant placement in anterior sites. The study sample included both male and female gender, predominantly south Indians. The necessary data such as age, gender, implant site, necessity of flap advancement, etc. was recorded. Incomplete patient data was excluded. Data was recorded in Microsoft Excel and exported to the statistical package of social science for Windows (SPSS) and subjected to statistical analysis. Chi Square test was used for comparison of groups.
Results
The sample size was found to be 2,116. The patients were split into 4 age groups. 16.19% were in the age group 18-30 years, 39.88% were in the age group 30-50 years, 40.39% were in the age 50-70 years and 3.56% were in the age group 70-90 years (Figure 1). 67.08% of the cases were males and 32.92% were females (Figure 2). 28.29% of the cases needed flap advancement, while 71.71% of the cases did not require flap advancement (Figure 3). 61.74% of the cases were maxillary anteriors and 38.26% of the cases were mandibular anterior (Figure 4). The correlation between Gender and requirement of flap advancement was not found to be statistically significant, meaning there is no correlation between gender and requirement of flap advancement (Figure 5). The correlation between Age and requirement of flap advancement was not found to be statistically significant, meaning there is no correlation between age and requirement of flap advancement (Figure 6). The correlation between implant site and requirement of flap advancement was not found to be statistically significant, meaning there is no correlation between the site of implant placement and requirement of flap advancement (Figure 7).
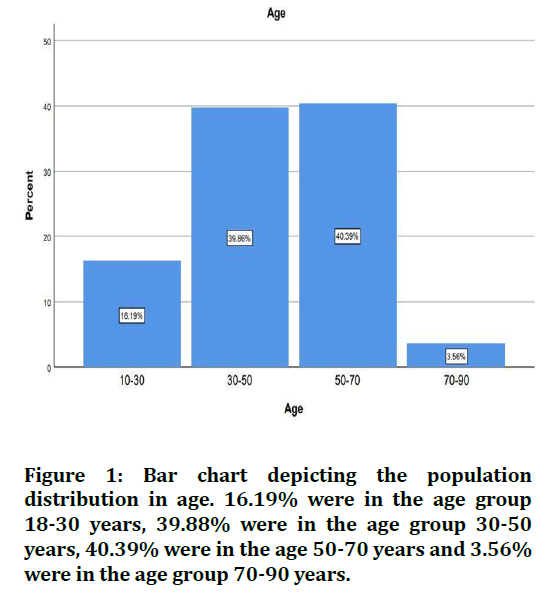
Figure 1. Bar chart depicting the population distribution in age. 16.19% were in the age group 18-30 years, 39.88% were in the age group 30-50 years, 40.39% were in the age 50-70 years and 3.56% were in the age group 70-90 years.
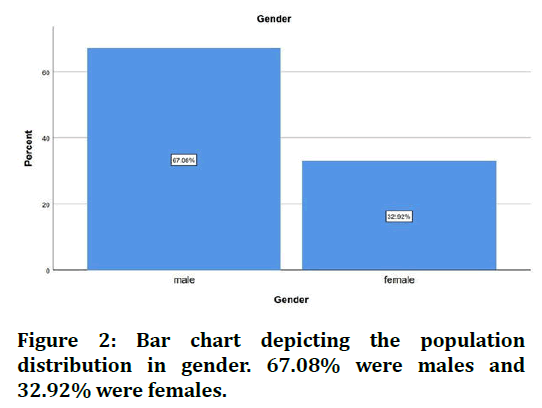
Figure 2. Bar chart depicting the population distribution in gender. 67.08% were males and 32.92% were females.
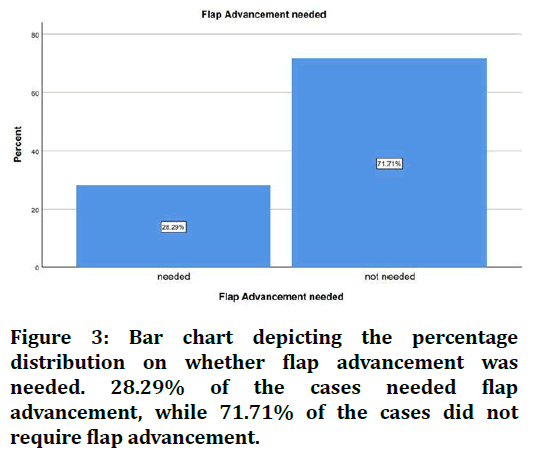
Figure 3. Bar chart depicting the percentage distribution on whether flap advancement was needed. 28.29% of the cases needed flap advancement, while 71.71% of the cases did not require flap advancement.
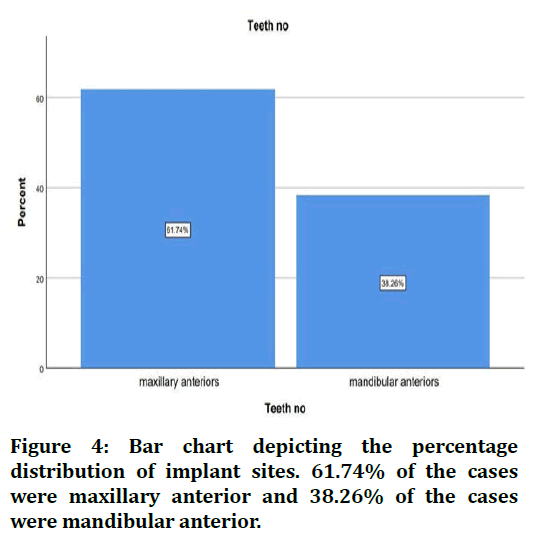
Figure 4. Bar chart depicting the percentage distribution of implant sites. 61.74% of the cases were maxillary anterior and 38.26% of the cases were mandibular anterior.
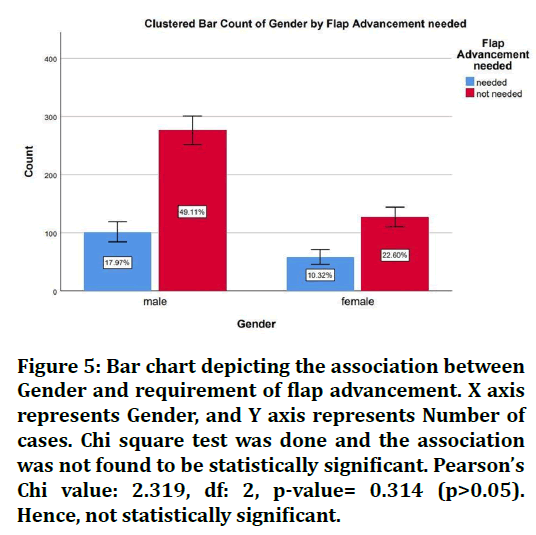
Figure 5. Bar chart depicting the association between Gender and requirement of flap advancement. X axis represents Gender, and Y axis represents Number of cases. Chi square test was done and the association was not found to be statistically significant. Pearson’s Chi value: 2.319, df: 2, p-value= 0.314 (p>0.05). Hence, not statistically significant.
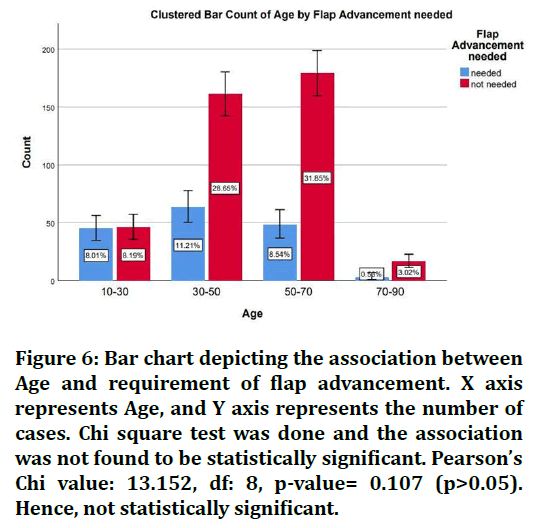
Figure 6. Bar chart depicting the association between Age and requirement of flap advancement. X axis represents Age, and Y axis represents the number of cases. Chi square test was done and the association was not found to be statistically significant. Pearson’s Chi value: 13.152, df: 8, p-value= 0.107 (p>0.05). Hence, not statistically significant.
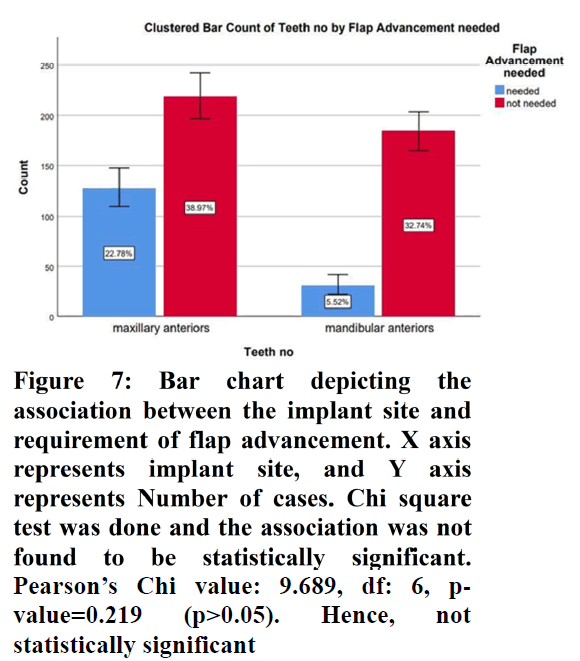
Figure 7. Bar chart depicting the association between the implant site and requirement of flap advancement. X axis represents implant site, and Y axis represents Number of cases. Chi square test was done and the association was not found to be statistically significant. Pearson’s Chi value: 9.689, df: 6, p-value=0.219 (p>0.05). Hence, not statistically significant
Discussion
In the current study, it can be concluded that there is no significant association between anterior implant placements. It is not dependent on the site of the implant. Previous study conducted by Zazou et al. concluded that flap advancement can be used in mandibular posteriors, especially if ridge augmentation is needed. This is used in Guided Bone Regeneration (GBR) [38]. The necessity for flap advancement has to be correlated with their pros and cons. Many studies have reported complications with advancement of flap. Complications have been documented in up to 25% of horizontal and 45.5 percent of vertical bone augmentation surgeries; as a result, various authors have established classification and management strategies for these complications [39].
Flap sloughing can occur as a result of a disruption in blood flow, resulting in tissue necrosis; therefore, careful flap management is critical during periosteal release to avoid excessive flap thinning or perforation. Damage to vascular and neurologic tissues are two further surgical consequences. Membrane contamination and infection, with or without bacterial contamination, are not uncommon, particularly with nonresorbable membranes [40]. Exposed bone graft particles and fixation are also prevalent. When doing a treatment like ridge augmentation, it's typical to underprepare the flap, which leads to tensionless closure failure. Primary closure will not be achieved if the tissue is not adequately released, and excessive force on the sutures will be required to seal the wound. Suture necrosis and dehiscence along the suture line are possible outcomes. To eliminate this problem, it's best to prepare the flap for advancement before putting bone grafts and barriers in place. The extent to which the tissue must be advanced is determined by the amount of the predicted enlargement in breadth and height. The buccal flap should be allowed to move 3 to 5 millimetres. The failure to provide tensionless closure is the most common cause of wound dehiscence. Infection, stress from opposing teeth, discomfort from a removable prosthesis, and hematoma formation are all possible causes of a dehiscence. A dehiscence might occur if a patient brushes on the sutures too soon. The chances of a successful augmentation are diminished if a dehiscence occurs. Attempting to restore a dehiscence is not a good idea. The clinician should allow it to cure naturally.
The viability of a flap is dependent on the flow of oxygenated blood to the flap's leading edges. As the distance from the feeding artery or arteriole rises, blood perfusion through the vascular plexuses diminishes [41]. The distal tip of an advancement flap is most vulnerable to necrosis because it has fewer blood vessels and is farthest from the feeding artery or arteriole, and the portion of the flap sutured under the greatest tension because the tension from the closure causes compressive forces on blood vessels [42].
Hence, it is understandable why clinicians debate on whether flap advancement is needed. But, it can be concluded that while many studies have shown the clinical outcome with flap advancement, it is more frequently indicated in complicated implant placements (like ridge augmentation). In cases of anterior implant placements, Flap advancement is usually not needed. But a true association cannot be made between the implant site and necessity for flap advancement, as flap advancement is decided based on various factors mentioned above.
Conclusion
Thus, it can be concluded that there is no association between anterior implant placement and flap advancement. Flap advancement is decided based on various factors, but the implant site alone is not determinative of the need for advancement of flap.
Acknowledgement
The authors are thankful to Saveetha Dental College for providing a platform to express our knowledge
Author Contributions
Santhosh Bala contributed to data collection, analysis and interpretation and drafting of the article. Abhinav RP, Subhashree, Thiyaneswaran contributed to the critical revision of the article
Conflict of Interest
No potential conflict of interest relevant to this article was reported
Source of Funding
The present study was supported by
Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Saveetha University, India
References
- Plonka AB, Sheridan RA, Wang HL. et al. Flap designs for flap advancement during implant therapy: A systematic review. Implant Dent 2017; 26:145-152.
- Spray JR, Black CG, Morris HF, et al. The influence of bone thickness on facial marginal bone response: Stage 1 placement through stage 2 uncovering. Ann of Periodontol 2000; 5:119-128.
- Brånemark PI, Hansson BO, Adell R, et al. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period . Scand J Plast Reconstr Surg 1977; 16:1–132.
- Greenstein G, Greenstein B, Cavallaro J, et al. Flap advancement: Practical techniques to attain tension free primary closure. J Periodontol 2009; 80:4-15.
- Simion M, Dahlin C, Trisi P, et al. Qualitative and quantitative comparative study on different filling materials used in bone tissue regeneration: a controlled clinical study. Int J Periodontics Restorative Dent 1994; 14.
- Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent 2006; 15:8-17.
- Melcher AH. On the repair potential of periodontal tissues. J Periodontol 1976; 47:256-260.
- Fu JH, Wang HL. Horizontal bone augmentation. The decision tree. Int J Periodontics Restorative Dent 2011; 31.
- Nevins M, Mellonig JT. Nevins M, et al. The advantages of localized ridge augmentation prior to implant placement: A staged event. Int J Periodontics Restorative Dent 1994; 14.
- Kalaivani N, Arun M, Abhinav RP, et al. Requirement of osteoplasty in dental implant surgery. A retrospective analysis. J Long-term Effects Med implants 2020; 30.
- Buser D, Dula K, Hirt HP, et al. Lateral ridge augmentation using autografts and barrier membranes: A clinical study with 40 partially edentulous patients. J Oral Maxillofac Surg 1996; 54:420-432.
- Gopal TM, Subhashree R, Nesappan T. et al. Association of gingival Biotype and soft tissue healing one week after implant placement. J Long Term Effects Med implants 2020; 30.
- von Arx T, Kurt B. Implant placement and simultaneous ridge augmentation using autogenous bone and a micro titanium mesh. A prospective clinical study with 20 implants. Clin oral Implants Res 1999; 10:24-33.
- Vergara JA, Quiñones CR, Nasjleti CE, et al. Vascular response to guided tissue regeneration procedures using nonresorbable and bioabsorbable membranes in dogs. J Periodontol 1997; 68:217-224.
- Park SH, Lee KW, Oh TJ, et al. Effect of absorbable membranes on sandwich bone augmentation. Clin Oral Implants Res 2008; 19:32-41.
- Fu JH, Oh TJ, Benavides E, et al. A randomized clinical trial evaluating the efficacy of the sandwich bone augmentation technique in increasing buccal bone thickness during implant placement surgery. I. Clinical and radiographic parameters. Clin Oral Implants Res 2014; 25:458-467.
- McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol 2007; 78:377–396.
- Duraisamy R, Krishnan CS, Ramasubramanian H, et al. Compatibility of nonoriginal abutments with implants. Evaluation of microgap at the implant–abutment interface, with original and nonoriginal abutments. Implant Dent 2019; 28:289-295.
- Anbu RT, Suresh V, Gounder R, et al. Comparison of the efficacy of three different bone regeneration materials. An animal study. Eur J Dent 2019; 13:022-028.
- Sekar D, Mani P, Biruntha M, et al. Dissecting the functional role of microrna 21 in osteosarcoma. Cancer Gene Ther 2019; 26:179–182.
- Sekar D. Circular RNA. A new biomarker for different types of hypertension. Hypertens Res 2019; 42:1824-1825.
- Bai L, Li J, Panagal M, et al. Methylation dependent microRNA 1285-5p and sterol carrier proteins 2 in type 2 diabetes mellitus. Artificial Cells Nanomed Biotechnol 2019; 47:3417-3422.
- Sivasamy R, Venugopal P, Mosquera E. et al. Synthesis of gd2o3/cdo composite by sol-gel method. Structural, morphological, optical, electrochemical and magnetic studies. Vacuum 2020; 175:109-255.
- Sekar D, Nallaswamy D, Lakshmanan G. et al. Decoding the functional role of long noncoding rnas Lncrnas in hypertension progression. Hypertens Res 2020; 43:724-725.
- Preethi KA, Lakshmanan G, Sekar D, et al. Antagomir technology in the treatment of different types of cancer. Epigenomics 2021; 13:481–484.
- Preethi KA, Sekar D. Dietary microRNAs Current status and perspective in food science. J Food Biochem 2021; 45:13827.
- Bakshi HA, Mishra V, Satija S, et al. Dynamics of prolyl hydroxylases levels during disease progression in experimental colitis. Inflamm 2019; 42:2032-2036.
- Ezhilarasan D. Dapsone-induced hepatic complications. It’s time to think beyond methemoglobinemia. Drug Chem Toxicol 2021; 44:330-333.
- Thakur RS, Devaraj E. Lagerstroemia speciosa (L.) Pers. triggers oxidative stress mediated apoptosis via intrinsic mitochondrial pathway in HepG2 cells. Environ Toxicol 2020; 35:1225-33.
- Shebi S, Ezhilarasan D, Thomas J, et al. Gracilaria foliifera (Forssk.) Børgesen ethanolic extract triggers apoptosis via activation of p53 expression in HepG2 cells. Pharmacogn Mag 2019; 15:259.
- Rajendran S, Annadurai G, Rajeshkumar S, et al. Characterization and toxicology evaluation of zirconium oxide nanoparticles on the embryonic development of zebrafish, Danio rerio. Drug Chem Toxicol 2018; 20:104-111.
- Balusamy SR, Perumalsamy H, Veerappan K, et al. Citral induced apoptosis through modulation of key genes involved in fatty acid biosynthesis in human prostate cancer cells. In silico and in vitro study. Bio Med Res Int 2020; 18:2020.
- Arvind P, Jain RK. Skeletally anchored forsus fatigue resistant device for correction of Class II malocclusions.A systematic review and meta analysis. Orthod Craniofac Res 2021; 24:52-61.
- Venugopal A, Vaid N, Bowman SJ. et al. After all, you may be another Choluteca Bridge. Insemin Orthod 2021; 27:53-56.
- Ramadurai N, Gurunathan D, Samuel AV, et al. Effectiveness of 2% articaine as an anesthetic agent in children. Randomized controlled trial. Clin Oral Invest 2019; 23:3543-3550.
- Varghese SS, Ramesh A, Veeraiyan DN. et al. Blended module based teaching in biostatistics and research methodology. A Retrospective study with postgraduate dental students. J Dent Educ 2019; 83:445-450.
- Mathew MG, Samuel SR, Soni AJ, et al. Evaluation of adhesion of Streptococcus mutans plaque accumulation on zirconia and stainless steel crowns and surrounding gingival inflammation in primary molars. Randomized controlled trial. Clin Oral Invest 2020; 24:3275-3280.
- Zazou N, Diab N, Bahaa S, et al. Clinical comparison of different flap advancement techniques to periosteal releasing incision in guided bone regeneration. A randomized controlled trial. Clin Implant Dent Related Res 2021; 23:107-116.
- Rocchietta I, Fontana F, Simion M. et al. Clinical outcomes of vertical bone augmentation to enable dental implant placement. A systematic review. J Clin Periodontol 2008; 35:203-215.
- Milinkovic I, Cordaro L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement. A systematic review. Int J oral Maxillofac Surg 2014; 43:606-625.
- Dahlin C, Lekholm U, Becker W, et al. Treatment of fenestration and dehiscence bone defects around oral implants using the guided tissue regeneration technique: a prospective multicenter study. Int J Oral Maxillofac Implants 1995; 10.
- Hämmerle CH, Jung RE, Yaman D, et al. Ridge augmentation by applying bioresorbable membranes and deproteinized bovine bone mineral.A report of twelve consecutive cases. Clin Oral Implants Res 2008; 19:19-25.
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Indexed at, Google scholar, Cross ref
Author Info
Santhosh Bala S1, Abhinav RP1*, Subhasree R2 and Thiyaneswaran N2
1Department of Oral and Maxillofacial Surgery, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, India2Department of Implantology, Saveetha Dental College and Hospitals Saveetha Institute of Medical and Technical Sciences, Saveetha University, India
Received: 09-Jan-2022, Manuscript No. JRMDS-22-51428; , Pre QC No. JRMDS-22-51428 (PQ); Editor assigned: 11-Jan-2022, Pre QC No. JRMDS-22-51428 (PQ); Reviewed: 25-Jan-2022, QC No. JRMDS-22-51428; Revised: 28-Jan-2022, Manuscript No. JRMDS-22-51428 (R); Published: 04-Feb-2022
