Research - (2022) Volume 10, Issue 3
Bacteriology of Diabetic Foot and Hand UlcersâA Preliminary Qualitative and Quantitative Analysis
Muhammad Musthafa Poyil* and Nadeem Bari
*Correspondence: Muhammad Musthafa Poyil, Department of Basic Medical Sciences, College of Medicine, Prince Sattam Bin Abdulaziz University, Saudi Arabia, Email:
Abstract
Diabetes mellitus is a disease which occurs when the pancreas either fails to secrete the insulin or the body becomes unable to use the secreted insulin. As the disease progresses, diabetic ulcers which are open sores or wounds may develop as it has been reported in more than 25% of the patients. Even though the diabetic foot ulcers are more common the ulcers can develop on hands also. Infections with various pathogenic bacteria are common in such ulcers (40%-80%) and the multi-drug resistant bacterial strains and the complication arise from them cause prolonged morbidity, amputation of lower limb and in extreme cases even mortalities. So, to understand the quantitative and qualitative bacterial population in such diabetic wound ulcers are important. In the present study we analyze 53 diabetic patients with foot or hand ulcers for the characteristics of the bacterial community in their ulcers. 43 (81.13%) patients were having diabetic foot ulcers and the remaining 10 (18.86%) patients were with diabetic hand ulcers. 90.69% of patients with diabetic foot ulcers and 70% of diabetic hand ulcer patients were males. 39.53% of the diabetic patients were graded as Wagner 2 and there was no any patient with Wagner grade–5. Different types of Gram positive and Gram negative bacterial pathogens were isolated and identified from both the types of ulcers and they included Staphylococcus aureus, Streptococcus spp., E. coli, Proteus spp., Pseudomonas spp., Klebsiella spp. etc. Thus this study underlines the seriousness of bacterial colonization in diabetic ulcers and tries to aware about the importance of treatment with rationale use of antibiotics and with proper management.
Keywords
Diabetes mellitus, Diabetic foot ulcer, Diabetic hand ulcer, Bacterial infections, Wagner wound grading
Introduction
Diabetes mellitus is one of the challenging, chronic and complex health issues worldwide, characterized by a number of metabolic complications including hyperglycemia and dyslipidemia due to the alterations in the metabolism mechanisms of carbohydrates, protein and fat [1], and the disease sets in when the pancreas fails to synthesize the insulin or when the system is incompetent to effectively utilize the produced insulin [2-4]. It has affected more than 425 million people across the globe according to a 2017 study itself [5], and at a high pace, the prevalence has been noted to be increasing with an estimated 592 million patients by 2035 [3]. The world health organization- WHO predicts that the adult mortality from the diabetes mellitus will be doubled by the year 2030 [6].
Following diabetes, blood supply to body parts becomes poorer resulting in the development of ulcers in body extremities like foot and, even though not that common, in hands too. If not treated and managed effectively, these ulcers are prone to fungal and bacterial infections [6,7]. The complications associated with diabetes mellitus is being worsened by the acute bacterial skin and skin structures infections (ABSSSIs) leading to prolonged morbidity, hospital expenditures and high rate of mortalities [8,9]. The ABSSSIs when caused by multidrug resistant (MDR) microorganisms, which are understudied [10], the clinical conditions get worsen. The hand infections in diabetic patients are usually underestimated but results in extensive destruction of tissues. 41% of hand infection cultures in diabetic mellitus patients proves the presence of poly-microbial populations and if left untreated, in case of deep infections, they could lead to increased morbidity, re-surgical procedures, amputations and even mortalities [11].
When uncontrolled, the diabetic mellitus patients develop complications that results latency in healing and increase the susceptibility for getting victimized for emerging infections mainly by bacterial pathogens [12]. Many of the acute infections in diabetic patients predominantly occur with Gram-positive, aerobic cocci in which Staphylococcus aureus and beta-hemolytic Streptococci would be the predominant members (5,13) and in chronic infection cases the causative agents could be aerobic, Gram-positive cocci or obligate anaerobic Gram-negative bacilli [5,14]. In urinary tract infection in diabetic patients the Gram-negative E. coli and Klebsiella pneumonia are the mostly encountered bacterial pathogens [14].
Materials and Methods
Sample collection
From the diabetic in-patient section of a tertiary hospital facility, swab specimens were collected from the foot and hand ulcers of 53 patients. In-charge clinicians evaluated the severity of lesions and ulcers, and graded according to the Wagner wound classification system [15]. All the selected 53 patients with diabetic hand and foot infections above 40 years of age.
Identification of the Isolates–Primary screening
The samples were subjected to basic biochemical (to ensure hyperglycemia) and detailed microbiological processes as per standard protocols. For the identification of the bacterial isolates the primary analysis like colony morphology, Gram’s staining, motility observation etc. were performed.
Identification of the Isolates–Biochemical tests
The biochemical tests like IMViC (Indole, MRVP, Citrate), Catalase, Oxidase, TSI, Nitrate, Urease sugar fermentation reactions etc. were performed as per standard protocols [16,17]. All the results were recorded and analyzed.
Identification of the isolates–Culture on different media
For the isolation of various suspected aerobic and anaerobic pathogen, media like nutrient agar, MacConkey agar, blood agar, cetrimide agar, mannitol salt agar, eosin methylene blue agar, brain heart infusion agar, Robertson’s cooked meat medium etc. were used and all the cultures were performed in triplicates. The inoculation, incubation etc. were performed as per standard procedures.
Results
A total of 53 patients, after confirming their hyper glycemic condition through biochemical analysis of the blood samples, were selected for the study. Out of them 43 (81.13%) patients were found to have developed diabetic foot ulcers and the remaining 10 (18.86%) patients were having diabetic hand ulcers. Among the patients with diabetic foot ulcers, huge majority of them (39 patients, 90.69%) were males and the remaining 4 (9.3%) patients were females. In the case of diabetic hand ulcer, out of the total 10 patients identified with, 7 (70%) were males and 3 (30%) were females. The average age of the patients with diabetic foot ulcers was found to be 59.77 and with diabetic hand ulcer was found to be 55.22.
While grading the diabetic foot wound based on their severity in accordance with the Wagner wound classification system, it was assessed as follows: Grade 0 – intact skin with o any abnormalities, Grade 1 is superficial ulcer of skin or subcutaneous tissue, Grade 2 – ulcers extend into tendon, bone, or capsule, Grade 3 – deep ulcer with osteomyelitis, or abscess, Grade 4 – partial foot gangrene and Grade 5 – whole foot gangrene. The results in the present study showed that there were 11 patients graded as Wagner 1 which constituted to 25.58% of the total patients with diabetic foot ulcers. Another 17 patients (39.53) were graded as Wagner 2 and this grade had the highest number of patients. Wagner 3 graded patients were only 3, and it comes 6.97%. Wagner 4 grade was the second largest grade with 12 patients and 27.90%. There were no patients with Wagner grade 5. The results are tabulated (Table 1) and shown in the graph (Figure 1).
| Patients Characteristics | ||
| Ulcer area | Foot | Hand |
| Total no. specimens | 43 | 10 |
| Extent of foot infection: number (percentage) | ||
| Wagner 1 | 11 (25.58) | 2 (20) |
| Wagner 2 | 17 (39.53) | 3 (30) |
| Wagner 3 | 3 (6.97) | 3 (30) |
| Wagner 4 | 12 (27.90) | 2 (20) |
| Wagner 5 | --- | --- |
Table 1: Wagner grading of diabetic foot and hand ulcers.
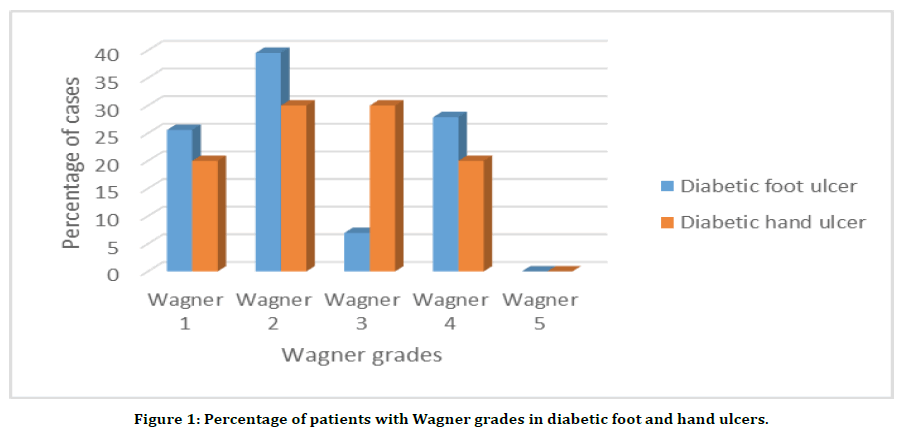
Figure 1. Percentage of patients with Wagner grades in diabetic foot and hand ulcers.
Coming to the qualitative and quantitative population of pathogenic bacteria isolated from the diabetic foot ulcers the Gram-positive Staphylococcus aureus was present in 27 of the 43 samples and the genus Streptococcus was present in 19 samples. The occurrence of various Gram-negative pathogenic bacteria was as follows: E. coli was present in 27 samples, Proteus spp. in 21 samples, Pseudomonas spp. in 11 samples and the Klebsiella spp. was present in 7 samples the results have been shown in Table 2 and in Figure 2.
| Name of the bacterial pathogens | Number of isolates |
|---|---|
| Staphylococcus aureus | 27 |
| Streptococcus spp. | 19 |
| Proteus spp. | 21 |
| Klebsiella spp. | 7 |
| E.coli | 27 |
| Psuedomonas spp. | 11 |
Table 2: Details of bacterial isolates form 43 diabetic foot ulcer samples.
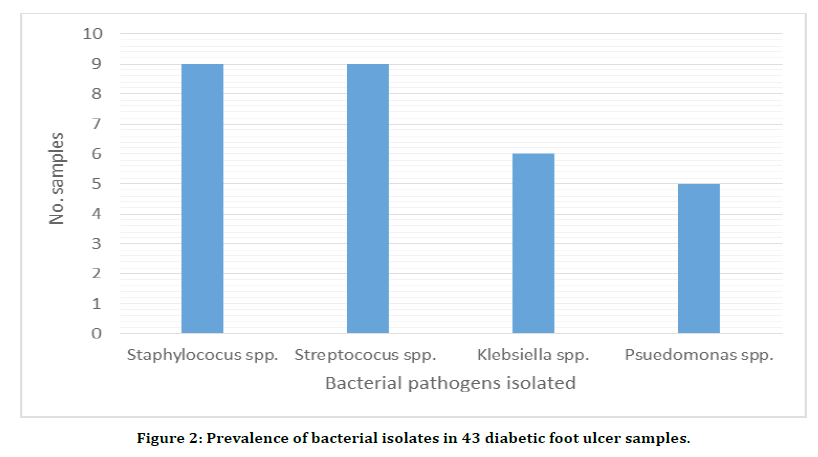
Figure 2. Prevalence of bacterial isolates in 43 diabetic foot ulcer samples.
The diabetic hand ulcer samples have also shown the presence of pathogenic bacteria. Out of the 10 samples, all the 9 had the presence of Staphylococcus spp. including Staphylococcus aureus and Streptococcus spp. six samples showed the presence of Gram-negative Klebsiella spp. and five samples had Pseudomonas spp. in them. The results have been shown in the Table 3 and in Figure 3.
| Name bacterial pathogens | No. isolates |
|---|---|
| Staphylococcus spp. | 9 |
| Streptococcus spp. | 9 |
| Klebsiella spp. | 6 |
| Pseudomonas spp. | 5 |
Table 3: Details of bacterial isolates form 10 diabetic hand ulcer sample.
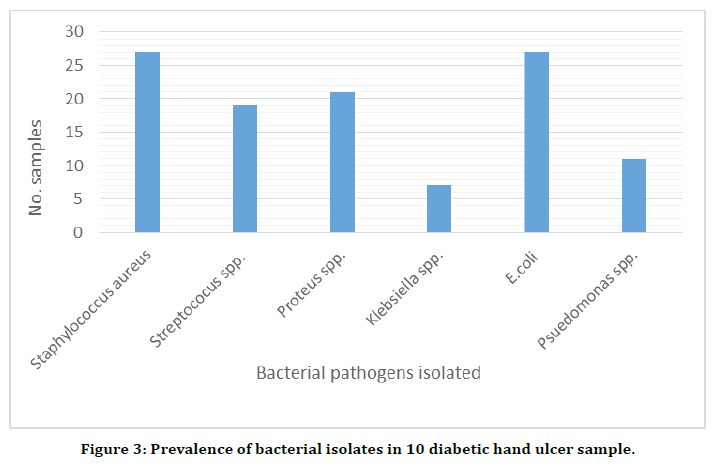
Figure 3. Prevalence of bacterial isolates in 10 diabetic hand ulcer sample.
Discussion
Diabetes mellitus is a chronic disease with a number of complications that arise when either the pancreas fails to secrete insulin or when the body fails to use the insulin that is being secreted. Development of ulcers and microbial infection worsen the condition further, and the spread of the pathogens to the bones and soft tissues of the body causes prolonged morbidity, situations like surgical amputation of lower limbs or even death [18]. In the present study the pathogenic bacterial population were isolated and identified from both the hand and foot ulcers of 53 in-patients of a diabetic facility of a tertiary hospital. The results showed the presence of the Gram-positive Staphylococcus aureus, Streptococcus spp. and Gram-negative pathogenic bacteria like E. coli, Proteus spp., Pseudomonas spp. and Klebsiella spp. The findings of this study are correlative with the reports by many researchers including that of Shahi et al. [19]. They concluded that the patients were infected with various bacterial pathogens including Staphylococcus spp., Enterococcus spp, Alcaligenes spp, Escherichia coli, Pseudomonas spp. etc. Another study by Anvarinejad et al. [20] reported that the Staphylococcus spp. and E. coli were the most isolated bacteria from diabetic ulcers. So the findings of this study, in general underlines the reports from other investigations.
Conclusion
Diabetes mellitus-the chronic disease caused by many defined and undefined factors makes the patient’s life miserable. The scenario worsens when the ulcers are formed on the body extremities and get infected with bacterial and fungal pathogens. In the present study 53 patients – with 43 having diabetic foot ulcers and 10 with diabetic hand ulcers were analyzed and found to have the pathogenic bacterial populations comprised of Staphylococcus aureus, Streptococcus spp, E. coli, Proteus spp., Pseudomonas spp. and Klebsiella spp. The notable thing to be noted is that, most of these pathogens have multi-drug resistant strains which could make the cases more terrible. So, it is important understand the species and strains of these bacteria and to examine their responses to different drugs and antibiotics. In short, the studies which are already going on across the globe need to be continued and evaluated.
Acknowledgement
The authors are grateful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia, for its support and encouragement in conducting the research and publishing this report.
Conflicts of Interest
Authors declare no conflict of interest.
References
- Kouitcheu Mabeku LB, Noundjeu Ngamga ML, Leundji H. Helicobacter pylori infection, a risk factor for Type 2 diabetes mellitus: A hospital-based cross-sectional study among dyspeptic patients in Douala-Cameroon. Sci Rep 2020; 10:1-1.
- Shaheen M, Al Dahab S, Abu Fada M, et al. Isolation and characterization of bacteria from diabetic foot ulcer: Amputation, antibiotic resistance and mortality rate. Int J Diabetes Dev Ctries 2021; 1-9.
- Katherine E, Macdonald, Sophie Boeckh, et al. The microbiology of diabetic foot infections: A meta-analysis. BMC Infect Dis 2021; 770:1-10.
- Schofield JD, Liu Y, Rao-Balakrishna P, et al. Diabetes dyslipidemia. Diabetes Ther 2016; 7:203-219.
- Ahmadishooli A, Davoodian P, Shoja S, et al. Frequency and antimicrobial susceptibility patterns of diabetic foot infection of patients from Bandar Abbas District, Southern Iran. J Pathogens 2020; 2020.
- Van Dieren S, Beulens W, Van der Schouw, et al. The global burden of diabetes and its complications: An emerging pandemic. Eur J Cardiovasc Prev Rehabil 2010; 17:S3–S8.
- Zhang P, Lu J, Jing Y, et al. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann Med 2016; 49:106–116.
- Marco Falcone, Juris JM, Maria GM, et al. Diabetes and acute bacterial skin and skin structure infections. Diabetes Res Clin Pract 2021; 174:108732.
- Critchley JA, Carey IM, Harris T, et al. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018; 41:2127–35.
- Carrillo-Larco RM, Anza-Ramírez C, Saal-Zapata G, et al. Type 2 diabetes mellitus and antibiotic-resistant infections: a systematic review and meta-analysis. J Epidemiol Community Health 2022; 76:75-84.
- Jalil A, Barlaan PI, Fung BK, et al. Hand infection in diabetic patients. Hand Surg 2011; 16:307-312.
- Chávez-Reyes J, Escárcega-González CE, Chavira-Suárez E, et al. Susceptibility for some infectious diseases in patients with diabetes: The key role of glycemia. Front Public Health 2021; 9:559595.
- Lipsky BA, Pecoraro RE, Wheat LJ. The diabetic foot: Soft tissue and bone infection. Infect Dis Clin North Am 1990; 4:409-432.
- Shah MA, Kassab YW, Farooq MJ, et al. Recent studies on urinary tract infections in diabetes mellitus. Health Sci J 2020; 14:724.
- Bessman AN, Wagner W. Nonclostridial gas gangrene: Report of 48 cases and review of the literature. JAMA 1975; 233:958-63.
- Cowan ST, Steel KJ. Manual for the identification of medical bacteria. Manual for the Identification of Medical Bacteria 1965.
- Pathare NA, Bal A, Talwalkar GV, et al. Diabetic foot infections: A study of microorganisms associated with the different wagner grades. Indian J Pathol Microbiol 1988; 41:437-47.
- Richard JL, Sotto A, Lavigne JP. New insights in diabetic foot infection. World J Diabetes 2011; 2:24-32.
- Shahi SK and Kumar A. Isolation and genetic analysis of multidrug resistant bacteria from diabetic foot ulcers. Front Microbiol 2016; 1464.
- Anvarinejad M, Pouladfar G, Japoni A, et al. Isolation and antibiotic susceptibility of the microorganisms isolated from diabetic foot infections in Nemazee hospital, Southern Iran. J Pathog 2015; 2015:328796.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Muhammad Musthafa Poyil* and Nadeem Bari
Department of Basic Medical Sciences, College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj, 11942, Saudi ArabiaReceived: 01-Mar-2022, Manuscript No. JRMDS-22-50877; , Pre QC No. JRMDS-22-50877 (PQ); Editor assigned: 03-Mar-2022, Pre QC No. JRMDS-22-50877 (PQ); Reviewed: 17-Mar-2022, QC No. JRMDS-22-50877; Revised: 21-Mar-2022, Manuscript No. JRMDS-22-50877 (R); Published: 28-Mar-2022
