Research - (2022) Volume 10, Issue 11
Chest Ultrasound
Ali Abdullah Alnakhli1*, Fatimah Taher Alnakhli2 and Maryam Abdullah Alnakhli2
*Correspondence: Ali Abdullah Alnakhli, EMS, Paramedic and MD, Szeged University, Szeged, Hungary, Email:
Abstract
Transthoracic ultrasonography (US) of the chest is advantageous for evaluating a number of peripheral parenchymal, pleural, and chest wall abnormalities. Increasing evidence regarding its importance in the field of pulmonary medicine is gradually coming to light, and therefore, it is understandable that its use has grown significantly among physicians in the past decades. For example, its use has dramatically increased in the intensive care units, as it may delineate abnormalities that might not be as obvious to our standard radiographic protocols. Unlike chest X-rays or physical examinations, ultrasound scanning of the thorax is considerably superior to detect critical disorders including pneumothorax, interstitial syndrome (edema), pulmonary consolidation (pneumonia), and pleural effusion. Effective therapeutic interventions, including thoracentesis, pleural effusion drainage with a closed tube, and transthoracic needle biopsy, can all be made safer and more effective with the use of ultrasound guidance. With no requirement for patient transport, ionizing radiation, or waiting time, the essential skill set is easy to learn, quick to execute, and repeatable. The use of point-of-care ultrasonography (POCUS) as a diagnostic tool for patients with acute dyspnea has increased significantly in recent years. A recent Sars-CoV-2 outbreak has led to an apparent shift in the usage of chest ultrasound as a screening tool for suspected cases of lung infection in emergency departments, where it has taken on the role of an iconic screening test. Ultrasound can be used to guide positive end-expiratory pressure settings, measure therapeutic efficacy, monitor the progression of the respiratory illness, and assist in the weaning process of the patient. This article discusses the fundamentals of ultrasonography and the numerous applications available. Three clinical instances constitute the heart of this essay, and they lead to discussions of diagnostic variability in pulmonary disorders as well as guidance for intervention. Finally, the third case leads us to address the COVID-19 outbreak as well as the significance of using ultrasound to diagnose the disease. Physicians may soon learn to employ lung ultrasonography as a diagnostic and monitoring tool in their practice. In time, the routine use of bedside chest X-rays appears to be becoming less effective. Computer tomography (CT) is an acceptable standard for 3D visualization of thoracic structures, whether for diagnostic or therapeutic purposes. However, moving a severely ill patient to a CT scan with all the supporting monitoring equipment may not always be a realistic choice. Chest ultrasonography has improved diagnostic accuracy while also improving quality and spatial resolution. With the emergence of patients in need of ventilation, chest ultrasonography has been recognized as an effective diagnostic tool. The advantages of chest ultrasound over other imaging modalities are real-time imaging, radiation-free imaging, and results that can be seen right away, among other things.
Keywords
COVID-19, Ultrasound, Emergency
Introduction
The Ultrasound (US)
As a precise amount of electricity is sent via the piezoelectric crystals in the ultrasound machine's transducer, the crystals receive the electric signal, which is then converted into mechanical energy, leading to the ultrasound wave production. Nearly all ultrasonic waves are reflected by the tissues or the nearby media because of the difference in the acoustic impedance of various tissues, which changes the ultrasound signal strength. Regardless of a few percentages of neglect of absorbed, refracted, transmitted, and attenuated waves, the ultrasonic signal intensity varies due to differences in acoustic impedance among tissues. Ultrasonic technology works by capturing sound waves and converting them to grayscale images, which may then be analyzed to learn more about the tissues being studied [1].
Ultrasonic wave propagation through various tissues and media is an active area of study. Echogenicity refers to a tissue's or medium's ability to generate echoes by transmitting ultrasonic vibrations in the context of surrounding structures. Because different tissues have different echogenicity’s, the contrast of the films that are created on the screen varies when ultrasonic waves pass through them. Lesion echogenicity can be compared to the liver and categorized as hyperechoic (white on the screen), hypoechoic (grey on the screen), anechoic (black on the screen), or isoechoic (the same color as the background, similar echogenicity to the liver) [1].
Types of transducers (Probs)
To perform chest ultrasound (CUS), linear arrays probe a high-frequency range between 5–12 MHz, providing good resolution but less penetration depth. A linear probe is optimal, especially to visualize pleural abnormalities. It is also helpful in ultrasound-guided vascular procedures [1]. It is rectangular, and the crystals are aligned in a linear fashion designed for superficial imaging. Curvilinear arrays probe a low-frequency range between 3–5 MHz, with good penetration depth but less resolution quality and a large sector width. The curvilinear probe is useful in assessing lung regions for consolidations, effusion, and visualization of the diaphragm. Curvilinear is preferred with large patients. Finally, a phased-array probe with a frequency range of 3–4.5 MHz is beneficial due to how easily it can be placed in the intercostal space footprint waves to demonstrate all the signs of Chest-US but with less image clarity. When pathology is present, a probe that can image both the surface and depth of the pleura and the lung parenchyma is essential. The use of a Micro-convex probe is recommended for this purpose owing to its large depth and frequency range, as well as the simplicity with which it may be utilized for intercostal scanning and access to the posterolateral lung field in a supine patient, as well as its ergonomic design (Figure 1).
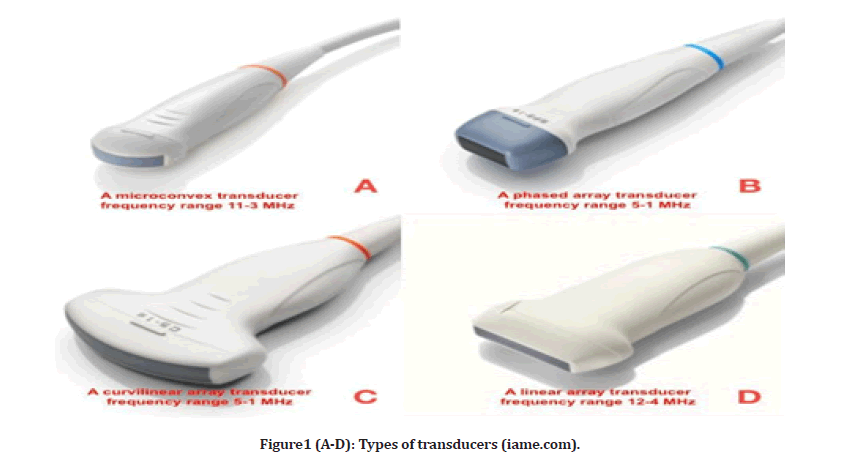
Figure1 (A-D): Types of transducers (iame.com).
Modes of US
While most ultrasound devices perform similarly, the designs of various manufacturers differ slightly. As a result, it is critical to familiarize yourself with the settings and controls of the individual equipment before starting scanning. When using an ultrasound machine, several controls can be used to fine-tune image quality. Most often, ChestUS is performed in B-mode or M-mode. Ultrasound imaging uses the B-mode or brightness mode, which is frequently referred to as grayscale or two- dimensional imaging because of the black and white image that is displayed on the ultrasound monitor. Moving objects can be seen well in M-mode, which is also known as motion mode. To illustrate motion in one-dimensional space, the horizontal axis shows time, and the vertical axis shows motion. It's possible to produce an M-mode image by placing the cursor over an object in the B-mode image and then activating M mode. M-mode imaging is an excellent tool for assessing cardiac valves and the activity of the fetus's heart. In terms of ChestUS, M-mode allows the clinician to analyze the respiratory variability of the inferior cava and, most importantly, to identify pneumothorax. Pleural mobility can be assessed using the M-mode, which uses a different type of imaging technique than the B-mode. Ultrasound Doppler is a feature that enhances ultrasonic imaging of blood vessels, reducing the risk of injury in an accident. Doppler sonography makes it possible to see the flow's direction and speed within a specific area.
The sonographer designates an area of interest, and the Doppler changes of the returning ultrasound waves are color-coded depending on their average velocity and direction [1].
Ultrasound for clinical applications
Blue protocols
To use ultrasound as a suitable bedside gold standard, the application demands knowledge of 10 signs, which score a diagnostic accuracy range of 90 percent to 100 percent [1]. Bedside Lung Ultrasound in Emergencies (BLUE) and the Fluid Administration Limited by Lung Sonography (FALLS) protocols are used in urgent care situations, which aims to reduce the usage of radiography or CT, lung ultrasound can be used to diagnose and treat acute respiratory failure and acute circulatory failure. Adults have been evaluated for these signs. There are specific profiles in the BLUE protocol for the most common medical conditions, such as pneumothorax, interstitial syndrome (edema), pneumonia, and pleural effusion (Figure 2). By monitoring for the change from A-lines to lung rockets, the FALLS protocols can identify clinical hypovolemia with an occlusion pressure of 18 mm Hg on the pulmonary artery. The FALLS protocols sequentially remove obstructive, cardiogenic, and hypovolemic shocks, enabling the discovery of distributive (usually septic) shocks more quickly [2]. These applications may be carried out with the aid of modest grayscale machines and a single micro-convex probe capable of scanning the whole body. Lung ultrasonography is a versatile technology that may also be used to reduce radiation doses in a variety of settings, from ARDS to trauma treatment, and from ICUs to points of care. If performed in appropriate places, training is the most significant constraint on the application of this type of visual medicine [1,2].
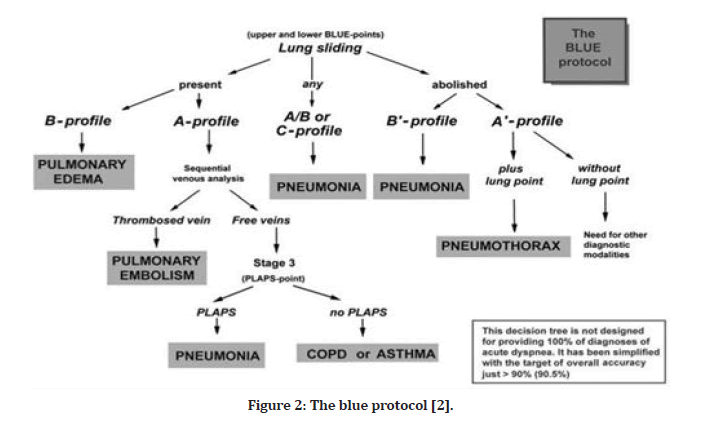
Figure 2: The blue protocol [2].
The 12 landmark zones of chest ultrasound scanning
Lung ultrasonography zones were split into three categories: The anterior axillary line split the anterior and lateral sides, while the posterior axillary line divided the lateral and posterior zones. From the sternal to the anterior axillary line, which corresponds to anterior zones 1 and 2. In between the two axillary lines are lateral zones 3 and 4 (axillary region). From the posterior axillary line to the spinal vertebrae, or posterior zones 5 and 6, respectively. For each hemithorax, six regions were divided into superior and inferior halves, resulting in a total of twelve regions to be studied [2]. (Figure 3).
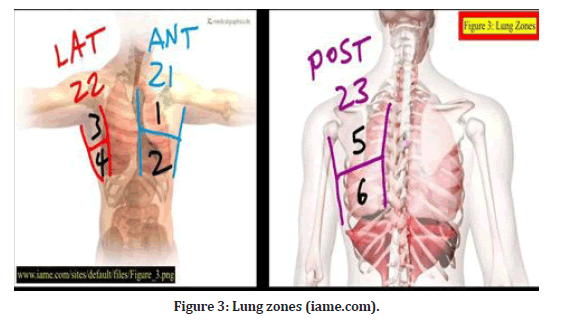
Figure 3: Lung zones (iame.com).
Dr. Lichtenstein's 7 principles
Prof. Daniel Lichtenstein is a visiting professor at the University Hospital Ambroise-Paré in Paris. Prof. Lichtenstein defined critical ultrasonography, which includes lung ultrasound, in 1991 as a comprehensive approach to the seriously sick with urgent therapeutic applications. Prof. Lichtenstein is the author of "Whole Body Ultrasound in the Critically Ill" (Springer, 2010). Springer published Lung Ultrasound in the Critically Ill in June 2015, which includes protocols for acute respiratory failure (BLUE), circulatory failure (FALLS), and cardiac arrest. (SESAME) (thoracentesis, subclavian venous line insertions, etc.).
One does not need a sophisticated ultrasound machine to carry out ChestUS. Artifacts are formed in the thorax as a result of gas and fluids being incompatible or being mixed due to pathologic conditions. The lung is the largest organ in the body in terms of volume and has a large surface area (about 1,500 cm2). As a result, exact boundaries must be specified. The pleural line is the origin of all lung symptoms. Because Chest US is mainly reliant on the analysis of created artifacts, this was previously addressed as a problem, indicating the infeasibility of ChestUS. Because the lung is a live organ, lung symptoms associated with the pleural line are dynamic. Because the majority of acute, life-threatening lung illnesses border the pleural line, ChestUS is a possibility. Almost all life-threatening illnesses, including those that are less obvious on other tests, will demonstrate an extensive location with ChestUS, such as a pneumothorax, which can be minor but still visible in a sufficiently large projection [1].
Dr. Lichtenstein's ten basic signs
There are 10 fundamental signs shown in Figure 4, beginning with the surface of the lungs (bat sign, lung sliding, A-lines), pleural effusions (quad and sinusoid effusions), lung consolidations (fractal and tissuelike signals), interstitial syndrome (lung rockets), and pneumothorax (stratosphere sign and lung point) [2].
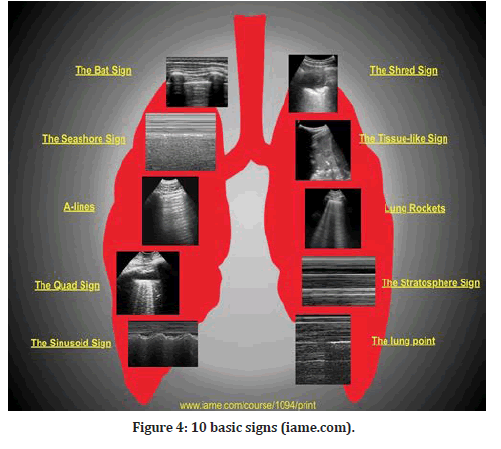
Figure 4: 10 basic signs (iame.com).
During the initial lung screening, the bat-wing sign, which is the baseline normal image, is acquired in B-mode (FIgure 4). A baseline image is essential for each ultrasound scan to correlate normal with abnormal. The pleural line is 0.5 cm below the neighboring upper and lower rib shadows, giving the impression of a bat wing, with the ribs serving as the wings [6]. All LUS signs arise from the pleural line. The exception is subcutaneous emphysema, which is an accumulation of air under the skin that will cause the qualities of ultrasonic waves to be abolished.
The A-lines (A-profile) are B-mode horizontal hyperechoic reverberation artifacts (FIgure 4). The distance between the transducer and the pleura is the same as the distance between the pleura and the first A-line, and so on for the second and third A-lines. The existence of the A-profile in the lungs confirms the presence of physiological free air [1].
Lung sliding is the third sign, and it is normal and dynamic, and it is acquired in B-mode and visualized at the level of the pleural line (Figure 4). As long as there is just around 50 mL of fluid in between the parietal and visceral pleurae, they can slide over one another during breathing. Lung sliding is synchronized with breathing, which reflects the normal physiological movement of the lung toward the chest wall. This corresponds to the seashore sign in M-mode, which reflects the relationship between lung sliding and superficial tissues [1].
The quad sign is the fourth sign; it is an aberrant static LUS sign shown in the B-mode that shows the presence of a pleural effusion. It is defined by four regular boundaries (FIgure 4). The ribs at the top and the parietal and visceral pleura at the bottom define the cephalic and caudal margins of a pleural effusion. The collection's deep limit is regular, nearly parallel to the pleural line, and is referred to as the lung line (i.e., visceral pleura). When the diaphragm is excluded from the picture, the pleural effusion appears rectangular (i.e., the quad sign), and when the diaphragm is visible, the basal lung may be seen floating in the irregular image of the effusion [1].
The sinusoid sign, which is an aberrant dynamic LUS sign observed in M-mode and suggests the existence of a pleural effusion, is the fifth sign to look for (FIgure 4). During inspiration, this indication is present when the lung line shifts closer to the pleural line than it was previously. It is possible to achieve the sinusoid sign when the M-mode is enabled by holding the cursor in the middle of the field, which is seen in the B-mode and contains a substantial volume of pleural fluid. A sinusoid sign confirms the clinician's observations that pleural effusion is present, but it does not necessarily imply that the pleural fluid is interfering with lung dynamics in all cases [1].
"Shred sign" is the sixth sign described, and it is an anomalous indication that can only be seen in B-mode. In the majority of situations, the shred indication indicates non- translobar consolidation [1]. When the boundary between consolidated and aerated lung is uneven, the shred sign is created, resulting in the formation of the fractal line, which is opposite to the lung line. This is a symptom of pneumonia that should be taken seriously. This is a suggestive sign of pneumonia (FIgure 4).
The seventh indication is the tissue-like sign, which is an aberrant sign that may be seen in B-mode when there is a translobar consolidation of the lung [1]. (FIgure 4). It is also known as hepatization of the lung when excess fluid fills the lung, giving it an echogenic appearance similar to that of the liver.
B-line/lung rocket/comet tail artifacts are an extremely critical, if not the most essential, sign. Long, hyperechoic vertical B-lines that begin at the pleural line and run the length of the ultrasound screen, from top to bottom, can only be seen in B-mode, and they completely cover up the typical A-lines (FIgure 4). Due to the significant variation in acoustic impedance, these aberrations are the consequence of air and water mixing in the alveoli and interlobular septa. Because of the juxtaposition of air and water, the sound waves ricochet back and forth between the septa, forming lines for each reverberation that combine to produce B-lines. B-lines are a strong indicator of alveolar-interstitial syndromes and pulmonary edema, respectively [1].
One of the three signs that point to a pneumothorax is the absence of lung sliding, which is an aberrant sign seen in the B-mode. Visceral pleura cannot be detected because of the presence of air in the pleural space and lung sliding cannot be observed. Absent lung sliding can be shown by tracing the pleural line between two adjacent ribs. If there is any pleural involvement (pneumothorax, pleural effusion, pleurodesis) or lung involvement, the usual back and forth movement of the pleural line with breathing will not be noticed [1]. The M-mode can also be utilized to verify that the lungs are not moving. Because there is no movement in the pleural space, the image in M-mode shows only one pattern of parallel horizontal lines above and below it. A barcode sign, or stratospheric sign, is a common nickname for this pattern, which has a striking similarity to a barcode (Figure 4).
The tenth sign is the lung point, an abnormality visible in M-mode. The lung point is the junction of the two pleural layers (Figure 4). The lung point is regarded as confirmatory for pneumothorax in the absence of lung sliding and B-lines. This location will be seen when the M-mode is activated, and the probe is kept in the area where the slide appears and disappears. The lung point denotes the point at which lung inflation and deflation occur [1].
Volume Sweep Imaging (VSI) and VSI protocol
Respiratory illness is a major source of morbidity in adults and the primary cause of morbidity in children. Without any prior experience, VSI performed VSI external body landmarks, saving each step as a cine clip that was subsequently interpreted by a professional. For abnormal chest radiography, chest ultrasonography has a sensitivity of 93 percent and a specificity of 91 percent, and for pneumonia, ultrasound has a sensitivity of 100 percent and a specificity of 93 percent for clinical diagnosis [3].
Pneumonia seems to be the leading cause of mortality in children under the age of five [4]. Billions of people, around two-thirds of the world's population, lack access to diagnostic imaging, causing respiratory illness to deteriorate. In a meta-analysis, ultrasound was shown to be a promising imaging technique with outstanding sensitivity and specificity, with sensitivity and specificity of greater than 90% [3]. Adding to that, the possibility of a normal life-case physical exam and the patient's past medical history with the doctor's experience would all significantly increase the percentage.
VSI (volume sweep imaging) is a defined protocol of ultrasound examination technique in which the image capture and analysis are completely separated. Each probing sweep is preserved as an MP4 format clip for subsequent evaluation, and the VSI protocol is performed in the same manner each time, based purely on external body landmarks. The University of Rochester Medical Centre put the VSI lung ultrasound protocol to the test in a prospective trial that lasted seven months (Figure 5). The goal of the study was to compare the VSI lung ultrasound protocol to the standard of care radiography, with the premise that lung VSI would acquire interpretation that was consistent with radiography, clinical diagnosis, and chest CT scan. Individuals without sufficient prior expertise may readily learn VSI and perform it after only a few hours of training. The methodology was learned in about an hour by rural employees in Peru, and they demonstrated error-free performance after only a few trials; the complete VSI evaluation takes around 10 minutes on average. Using only three isolated locations in each hemi thorax, the previously disclosed lung FOCUS procedure has a diagnostic accuracy of over 90% for chest illnesses such as pneumothorax, pneumonia, and pleural effusion. VSI is a more comprehensive evaluation that should be at least equivalent [3].
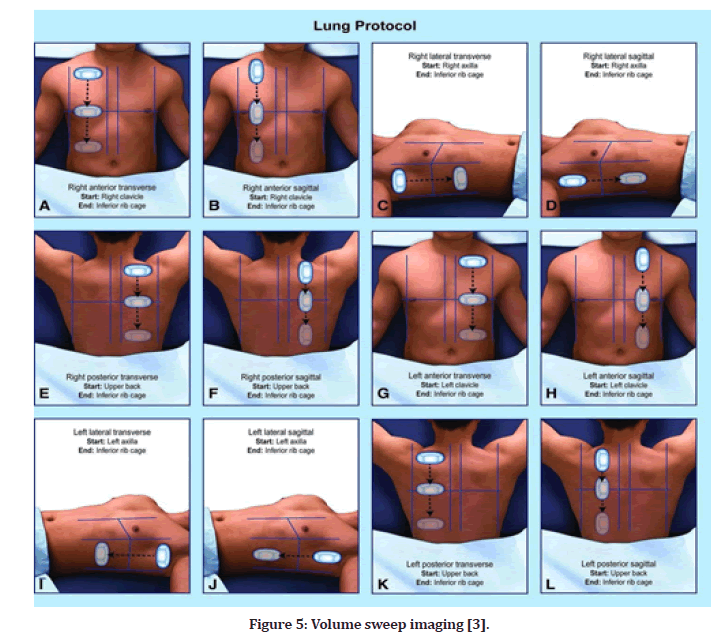
Figure 5: Volume sweep imaging [3].
The study concluded that x-ray and VSI imaging had roughly equivalent sensitivity and specificity for pneumonia [3]. The cine clips were rated as having adequate image quality by the readers. VSI has the potential to be an accurate, low-cost, and radiation-free method for diagnosing respiratory disorders, and it is equally as effective as a chest X-ray. VSI did not miss any cases of acute pneumonia that may have been clinically significant. Lung VSI has numerous potential public health applications, as well as the ability to view heart failure and pleural effusion. To enhance the variability of instances in this study, pediatric and adult patients were included. The purpose was to develop a proof of concept. Millions of people worldwide lack access to diagnostic imaging. The combination of lung ultrasound VSI and teleultrasound offer a promising low-cost approach to diagnosing a variety of respiratory disorders without the requirement for an expert sonographer [3]. USguided interventional procedures (thoracentesis, close tube drainage, TNB, FNA) Transducer design, signal processing, and Doppler technologies have all enhanced ultrasound imaging quality. To evaluate lesions of the chest wall, pleural cavity, peri-the diaphragmatic region, mediastinum, and peripheral lung, Transthoracic US can now diagnose a much wider spectrum of chest diseases. It improves the diagnostic potential and safety of invasive interventions. Many interventional techniques, such as thoracentesis, closed tube drainage for pleural effusions, and needle pleural biopsy, benefit from US guidance [5]. With real-time US monitoring and accurate puncture-guiding equipment, the US can guide TNB and other invasive thoracic interventions. Although US-guided TNB takes some experience, it is a reasonably simple procedure to master and may be utilized in a variety of scenarios where computed tomography-guided aspiration or biopsy was previously employed. Our experience with transthoracic ultrasound shows that it improves patient care, is efficient and costeffective, and has become a vital imaging tool in modern chest medicine [5].
Thoracentesis with ultrasound guidance and closed tube drainage for pleural effusion
When a pleural effusion of uncertain cause is suspected, the fluid must typically be obtained for cytologic, biochemical, or microbiologic investigation. By using ultrasound, the most accessible location of fluid buildup may be discovered, and the needle penetration depth can be measured. When the effusion is small or loculated, a time-consuming radiographic investigation is not possible, or a safe thoracentesis is required in a critically ill patient, a US-guided thoracentesis is an excellent option [5]. Direct observation of the effusion during thoracentesis is also possible with real-time imaging. These US-guided methods aid in increasing thoracentesis success rates and minimizing the risk of complications such as pneumothorax. Several clinical circumstances require the use of closed tube drainage for effusion. Massive effusions compromise the respiratory system, difficult parapneumonic effusions and empyema, haemothorax, and malignant effusions that prepare for pleurodesis [5]. Drainage-related complications, such as lung, diaphragm, liver, and spleen laceration, might be catastrophic. In cases of loculated effusion, improper tube placement may lead to drainage failure [5].
Pulmonary lesions can be diagnosed with an ultrasound-guided needle biopsy (TNB) and histopathology
For cytology and microbiology as well as histology, chest ultrasound can be utilized to guide TNB of the chest wall, peripheral lung lesions, or mediastinal lesions. A detailed discussion of the advantages and disadvantages of fine-needle aspiration vs. large-bore biopsy is outside the scope of this paper [5]. Clinical diagnosis for the majority of patients with primary lung cancer may be as straightforward as the fine-needle aspiration for cytologic assessment. On the other hand, non-specific, benign, or unfavorable consequences cast doubt on the data's validity. A negative fine-needle aspiration test does not exclude the possibility of malignancy. A suitable histological specimen is frequently required for benign lesions, mediastinal masses, and some malignancies, such as lymphomas and sarcomas [5].
Thoracic ultrasonography is a safe procedure that has higher diagnostic accuracy than fine-needle swabs. All patients referred for US-guided TNB should have grayscale and color Doppler conducted to identify the exact location, sonographic pattern, and blood flow information of the lesion [5]. When a lesion is shown to be TNB vulnerable, extreme caution is needed to prevent puncturing the aerated lung, major arteries, or main bronchi. While performing TNB, an echogenic focus may be shown on the real-time US display, allowing for exact needle advancement to the target lesion. The ribcage of a patient may conceal tiny peripheral lung nodules, which can constantly move when breathing. As a result of these concerns, TNB may become challenging. By utilizing real-time US surveillance, TNB can be performed at a time when the nodule is most easily accessible. This technique has been successfully utilized to find and biopsy tumors as small as 1 cm in diameter [5]. TNB can be successfully used to address the majority of large lung tumors. As a result, the majority of large lung tumors have extensive necrosis in their centers, leaving just a small section of the tumor alive. A biopsy or aspiration of a malignant tumor's necrotic core may provide a false negative result. By delineating the solid tumor portion and directing sampling of these mural regions, US guidance can assist in boosting TNB diagnostic yield [5].
It is possible to sample a lung tumor that is not in touch with the pleura but does have an acoustic window. The usual approach for the diagnosis of lung cancer and obstructive pneumonitis is fiberoptic bronchoscopy. In contrast to extraluminal lesions, fiberoptic bronchoscopy may only display external compression, and neither bronchoscopic biopsy nor transbronchial needle aspiration gives sufficient diagnostic information to confirm the diagnosis. These patients may have USguided transnasal biopsy to ascertain the histology of the obstructive tumor [5]. Through the consolidated lung, ultrasonography can visualize the core tumor and the nearby consolidated lung. Even though some blocking tumors are deeply buried and close to the hilum, enough specimens for cytologic or histologic diagnosis can be found. The needle can be guided around the consolidated lungs, major bronchi, and pulmonary arteries, minimizing the potential for serious hemorrhage. Ultrasound examination of mediastinal masses followed by US-guided biopsy is a rapid and safe method of obtaining histology specimens [5]. Transthoracic ultrasound demonstrates excellent discrimination between great vessels and target mass, particularly when color is used.
Ultrasonography-guided fine-needle aspiration (FNA) for lung infection microbiology and cytology evaluation
Transthoracic ultrasound can be used to evaluate isolated pulmonary infiltrates and to guide fineneedle aspiration for the detection of microbiologic lung infections [5]. Due to the fluid-filled air gaps in pulmonary consolidations, transthoracic ultrasound can identify deep-seated lesions. The presence of parapneumonic effusion, the formation of an abscess, or the presence of a tumor-associated with obstructive pneumonitis can be seen clearly. A trustworthy and non-contaminated transthoracic fine-needle aspirate for staining and culture of microorganisms. Real-time high-resolution ultrasound can identify the lesionpleura symphysis, preventing pneumothorax and abscess material leakage into the pleural cavity during needle aspiration. Additionally, ultrasound helps direct the needle point to the abscess fluid section, ensuring adequate fluid aspiration for bacterial culture. Cultures obtained using US-guided abscess aspiration outperform those obtained using sputum, blood, or broncho-alveolar lavage fluid [5]. A similar technique can be used to drain abscess fluid if clinically indicated.
Pulmonary cryptococcosis is a common infection that can affect both immunocompetent and immunocompromised individuals. A positive culture and a particular histopathologic investigation are required for a definitive diagnosis of pulmonary cryptococcosis. In such circumstances, serum cryptococcal antigen is frequently negative. As a result, diagnosing pulmonary cryptococcosis in a timely and accurate manner is frequently difficult. It is important to note that because the lesions pulmonary cryptococcosis tends to be subpleural, and specimens for fungal isolation can usually be acquired using ultrasound-guided needle aspiration. Transthoracic fine-needle aspiration guided by ultrasound through a consolidated lung is a relatively safe procedure. The high-resolution US in conjunction with color Doppler ultrasound can identify the consolidation's primary bronchi and arteries [5].
Pleural and parenchymal diseases pneumothorax
A significant sonographic symptom of pneumothorax is a lack of breath-dependent lung sliding on the real-time US, which occurs as a result of free air accumulating within the pleural cavity [5]. The presence of reverberation artifacts can be more prominent in minor pneumothoraxes, but they can disappear when the pneumothorax grows in size. Alternatively, the appearance of lung sliding or comet-tail artifacts rules out pneumothorax. The "curtain sign" is the downward displacement of the air-fluid level during inspiration, which enables the confident diagnosis of hydropneumothorax [5].
Although a pneumothorax can normally be detected on a chest radiograph, a minor pneumothorax may be missed on a radiograph of a supine patient. It is challenging to interpret radiographs taken in the critical care unit because artifacts and extensive lung alterations might mask or imitate pneumothorax. The following are the primary sonographic signals used to diagnose pneumothorax [6].
Lung sliding is not visible.
Horizontal artifacts are exaggerated.
The vanishing of comet-tail artifacts.
A widening of the pleural line to make it into a band.
Bedside sonography can be used to rule out pneumothorax [6]. The absence of lung sliding combined with the removal of comet-tail artifact has been reported to have a sensitivity of 100 percent and a specificity of 96.5 percent [6]. The use of ultrasound was shown to be more sensitive than radiography in the identification of pneumothorax and was second only to percutaneous lung biopsy in this regard. The absence of lung sliding should not be used as the sole criterion for diagnosing pneumothorax. In the absence of pneumothorax, lung sliding may be absent in individuals who have already had pleurodesis, have widespread pleural thickening associated with asbestos, or have adult respiratory distress syndrome.
Pulmonary edema/Congestion heart failure
When caring for heart failure patients, lung ultrasound is a vital skill to have. It can be utilized as an add-on to standard transthoracic echocardiography and is quite simple to perform. A thorough understanding of ChestUS enables the doctor to make an accurate diagnosis of pulmonary congestion and can aid in the assessment and treatment of heart failure patients who present with pulmonary congestion in the early stages. The acoustic mismatch between the lung tissues diminishes as pulmonary congestion and aeration loss worsen. This results in vertical reverberation artifacts referred to as "B- lines," They are only an expression of lung aeration loss; they are not pathognomonic for lung congestion. Chest US is a valuable tool for follow-up improvement of pulmonary congestion during diuretic treatment. When there are many B-lines, they tend to merge and form well-distinct, hyperechogenic, confluent zones. The presence of pleural effusion could also be a sign of progressive lung congestion. Very severe lung congestion or pulmonary edema can all induce an extensive loss of pulmonary aeration. A transudate (observed in pulmonary congestion) is always anechogenic.
The term "comet-tail" refers to a sign that is best described by several comet-tails flaring out from the pleural line. They are made from water-thickened interlobular septa and are suitable for non-radiologic bedside interstitium pulmonary edema estimation. The feasibility and utility of ultrasonography comet signs were investigated in 121 hospital admissions patients (43 females and 78 males; ages 67–12 years) admitted to a cardiology-pneumology combination ward using commercial echocardiographic machines [7]. The right and left chests of each subject were scanned using pre-set spots in numerous intercostal regions. The lung comet score of a patient is obtained by adding the number of comets in each scanning location. Within a few minutes, patients had a chest x-ray, with two pneumologist-radiologists blinded to clinical and echo data assessing the extravascular lung water score specifically. There was a linear connection between echocardiographic comet scores and radiologic extravascular lung water scores in 121 consecutive hospitalized patients. As a result, the comet-tail is a simple, non-time-consuming, and reasonably accurate chest ultrasonography indication of extravascular lung water that may be obtained at the bedside (also with portable echocardiographic equipment) and is not limited by cardiac acoustic window constraints [7].
Abscess and pneumonia
Pneumonia caused by pyogenic organisms can progress to necrosis, resulting in the development of a lung abscess. If an ultrasound reveals a hypoechoic lesion with a welldefined as well as an irregular wall, it is likely to be a lung abscess. Although the abscess's core is typically anechoic, it may include internal echoes and septations. The most common cause of pulmonary consolidation is nonspecific pneumonia [8]. Consolidation that resembles pneumonia on ultrasonography can be caused by infarction, hemorrhage, vasculitis, lymphoma, and bronchoalveolar cancer. A lung biopsy can be guided by the US if the diagnosis is unclear. This is particularly beneficial in immunocompromised individuals, for whom pulmonary consolidation may present a diagnostic challenge. As the infection advances, pneumonia's echogenicity grows and becomes more distinct. Successful treatment results in more air-inlet artifacts and a smaller pneumonia region due to regained ventilation inside the consolidation.
In healthy people, ultrasound waves are nearly completely reflected by the chest wall due to the substantial acoustic impedance differential between the chest wall and the air within the lung. However, in lung diseases that spread to the pleural surface, the replacement of air in the lung creates an acoustic window that allows for the examination of lung tissue. Lobar pneumonia, pleural segmental pneumonia, and pleural-based consolidation are all kinds of pneumonia that ultrasonography may identify. Several different forms of pneumonia may be detected. Pneumonia seems smaller on a US scan than it does on an X-ray. The lung appears diffusely echogenic during the initial stages of consolidation, mirroring the liver's sonographic texture. Pneumonia barely has a well-defined form, frequently exhibiting uneven or serrated edges. Branching echogenic structures are frequently found within pneumonia (87% of patients) and indicate air bronchograms. In cases of bronchial blockage, a fluid bronchogram is visible, which might be caused by either accumulated secretions or a proximal tumor. Even while the fluid bronchogram can be detected in isolated pneumonia, its presence in the right clinical setting should raise the suspicion of post-obstructive pneumonitis. Colour Doppler ultrasonography can assist in identifying uncomplicated pneumonia from postobstructive pneumonia by demonstrating normal or enhanced flow in the normal arteries of the consolidated lung. In instances of obstructive pneumonia, the pulsatility index of blood flow in the consolidation is greater than in the general population.
Pleural effusion
Pleural effusions may be detected with ultrasound, which is a very sensitive approach. Pleural effusion is characterized as a hypoechoic area between the parietal and visceral pleurae that changes shape in response to respiration [9]. Both free-flowing and confined fluid effusions can occur. The appearance of a tongue-like structure within a large effusion implies the existence of atelectasis as a result of lung compression. Effusions can be characterized as either anechoic, complex non septated, complex septated, or homogeneously echogenic based on the amount of echogenicity they generate. Between the visceral and parietal pleurae, an anechoic effusion lacks echogenic material. While a complex non septated effusion may have echogenic components, a complex septated effusion may contain floating strands or septa. Furthermore, a homogeneously echogenic effusion has a pleural space that is homogeneously echogenic as well. Sonographically, transudate, and exudate are distinguished by their sonographic characteristics. According to the findings of a study that included 320 participants, a transudate is always anechoic, although an anechoic effusion can be either a transudate or an exudate, and the other three types of pleural effusions are always exudative, according to the study. Typically, an effusion associated with a thickened pleura indicates an exudative condition. Consolidation of the lungs may occur as a result of infection-related exudates, and pleural nodules have been seen in patients with malignant effusion [5].
The Air/Fluid Ratio Principle is illustrated in Figure 6. Pulmonary sonogram results demonstrate a spectrum of air-to-fluid ratios, with air on the Y-axis and fluid on the X-axis, indicative of lung dry-to-wet spectrum abnormalities. For example, in a pneumothorax, the ultrasonic beam produces A-lines without lung sliding because it is passing through 100% air. When a beam is transmitted through 100% fluid, as is the situation with pleural effusion, quad and sinusoid signs are produced. As the beam travels through 98% air, it produces the usual lung A-line sign when seen with the lung sliding sign. The beam travels through 95% air (interstitial syndrome), resulting in numerous B- lines (lung rockets), and 10% air (or 90% fluid), resulting in signs of consolidation. The red arrow (on the right upper corner of Figur 6) indicates the deterioration of the lung as it transitions from dry (air) to wet (fluid), while the white arrow indicates the improvement of the lung as it transitions from wet to dry (Figure 6).
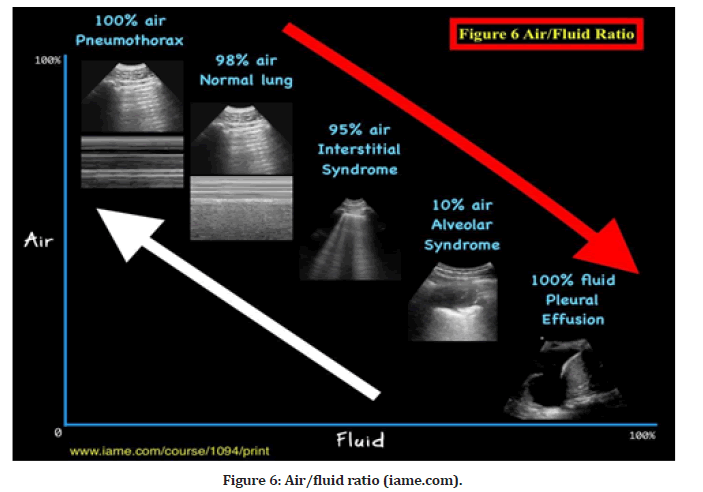
Figure 6:Air/fluid ratio (iame.com).
COVID-19 Outbreak
Chest US for improved outcome and reduction of complications
Since the outbreak of COVID-19, which has placed a substantial burden on the health system, the increased demand for patient imaging diagnostics has necessitated the invention of a fast, accurate, and minimally spread the infection-type machine. CXR is rapid but inaccurate; CT is precise but takes a long time between transport, waiting, and doing the test. Chest US, on the other hand, is very precise, fast, easy to clean, and convenient for bedside use. Chest US is beneficial for the management of ventilated patients, from the confirmation of endotracheal intubation to the commencement and prediction of successful weaning. This was determined after doing a review of previously published research conducted in intensive care units [1]. Chest US can be used as an imaging tool to monitor lung capacity during mechanical ventilation, thereby reducing the risk of barotraumas associated with ventilators and avoiding the injuries that can occur as a result.
COVID-19 Recommendations based on key revelations: When imaging is obtained conventionally, it is obvious that CT is the most accurate diagnostic technique for COVID-19 foci (pnuamonia). The patient is supine, which implies that the anterior surface is usually clear, whereas the posterior surface, particularly the lower field, is filled with exudate. It may show that the pleural line is irregular, interrupted, and/or discontinuous in morphology. Lesions in the subpleural space manifest as patchy, stripy, and/or nodular consolidation. The interstitial tissues involved have developed localized thickening and edema. From the pleura, fused B lines are apparent, as are B lines at fixed positions. An air-bronchogram can be visible within the consolidation. Ultrasound with color Doppler flow imaging (CDFI) demonstrates insufficient blood supply to the lesions. Localized pleural effusion is present around the lesions [10].
Changes in the lung parenchyma begin in the distal areas of the lung and move proximally with COVID-19 disease development. In the early stages, computed tomography imaging shows alterations in ground glass and opacities, followed by greater consolidations in the basal lung areas later in the illness course. The right middle and lower lobes are most usually affected, followed by the left upper lobe. Interstitial thickening, gravity consolidation, and alveolar destruction are all hallmarks of COVID- 19 pneumonia histopathology in the distal lung. Lung ultrasonography is an ideal imaging modality for tracking the course of COVID-19 pneumonia disease development (Figure 7).
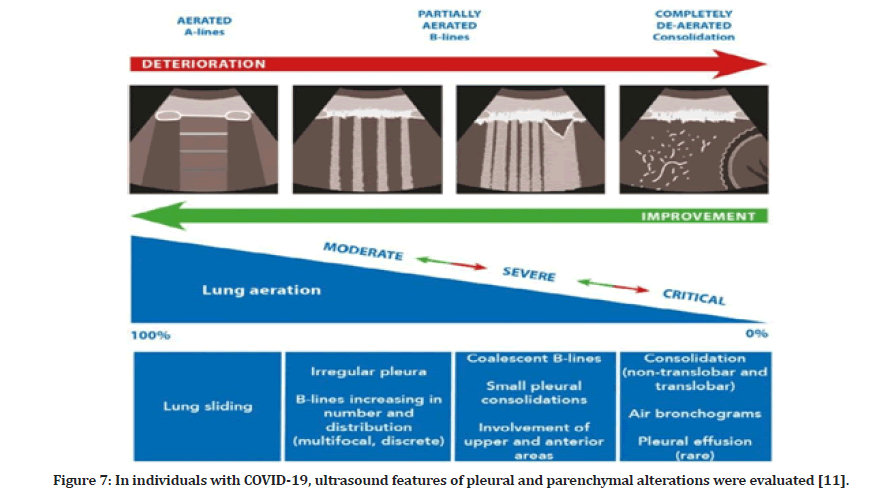
Figure 7: In individuals with COVID-19, ultrasound features of pleural and parenchymal alterations were evaluated [11].
Dr. Yale Tung Chen, who acquired coronavirus from a colleague while treating patients in Madrid, speaks with CNN's Alisyn Camerota about his experience under the headline "Doctor infected with coronavirus shares his story." Dr. Chen is board-certified in emergency medicine and internal medicine at the Hospital Universitario La Paz. From March 9 to April 17, 2020, he kept a COVID journal and utilized Twitter to discuss his COVID-19 learning experience. He had a sore throat, a strong headache, a dry cough, sudden shivers, and a general feeling of fatigue, although these were minor symptoms. Dr. Chen supplied clips of daily lung ultrasound (POCUS) scans, which revealed a minor pleural effusion in both posterior lower lobes. When symptoms continued to worsen, LUS revealed consolidations in the left subpleural space. Lung sliding persisted throughout the illness. In the lateral and posterior zones, the B-line signs were widespread bilaterally, but an A-line pattern was seen in the anterior zones. To begin with, Ibuprofen 600 mg and paracetamol 1 g were given as supportive treatments, to begin with (both three times a day). For seven days, Hydroxycholoroquine 200mg twice daily and azithromycin 250mg once per day were taken (off-label use). After two weeks, the patient's health improved significantly [12].
Clinical case 1: COVID-19 pneumonia 1 (Figure 8A to Figure 8F)
Figure 8A to 8F: X-ray and CT scans of COVID-19 pneumonia Imaging.
A COVID-19 pneumonia patient on COVID-19 regimen treatment
Date of admission: August 5, 2020, at 9:40 a.m.
Three days before his admission to the hospital on August 5, 2020, the patient had a COVID-19 PCR test that returned a positive result, and he was self-quarantined at home during that period. A COVID-19 infection was identified in a 55-year-old male hypertensive patient who presented with considerable shortness of breath, coughing, and fever. Increased oxygen demand necessitated the patient's transfer to the critical care unit (ICU). The blood pressure was 140/80 millimeters of mercury, the heart rate was 80 beats per minute, the body temperature was 37 degrees Celsius, and the oxygen saturation level was 97 percent. If the patient's health worsens, his physicians have determined that he will be intubated and placed on mechanical ventilation. Lung X-rays reveal bilateral infiltrations. Significant hilar shadows may be seen on both sides of the enhanced basal broncho vascular markings. On CT examination, bilateral diffuse veiling opacities are found. Both costo-diaphragmatic recesses are completely clear. Radiographs did not show any signs of lymph node growth in the hilar or mediastinum.
Labs: ESR: 34.0 mm/h,
Ferritin: 1951.40 ng/ml,
Fibrinogen: 920.0 mg/dL,
Platelets: 518 x 109/L,
Troponin I: 12.30 pg/dL,
Platelets: 518 x109/L,
Urea: 27.82 mg/dL,
C.R.P: 227.7 mg/L,
Na: 139 mEq/L,
K: 4.7 mEq/L
In a study utilizing a 12-zone scanning approach, lung ultrasonography was performed on 20 individuals with COVID-19. The following characteristics of the lungs were observed: thickening and irregularity of the pleural line. B-lines are not a fixed pattern; variations vary by patient and may be focal, multi-focal, or confluent. Also, consolidations happen in a variety of ways with the appearance of mobile air bronchograms. During the recovery phase, A-lines reappear, and the lung returns to its dry spectrum state. Pleural effusions are a relatively rare event. Alveolar interstitial patterns were found in the research group, ranging from mild to more severe bilateral interstitial patterns to lung consolidation. Table 1 illustrates what lung ultrasonography and chest CT look like in individuals with COVID-19 respiratory disease [13].
| Lung CT | Lung ultrasound |
|---|---|
| Thickened pleura | Thickened pleural line |
| Ground glass shadow and effusion | B lines (multifocal, discrete, or confluent) |
| Pulmonary infiltrating shadow | Confluent B lines |
| Subpleural consolidation | Small (centomeric) consolidations) |
| Translobar consolidation | Both non-translobar and translobar consolidation |
| Pleural effusion is rare. | Pleural effusion is rare |
| More than two lobes affected | Multilobar distribution of abnormalities |
| Negative or atypical in lung CT images in the super-early stage, then dif-fuse scattered or ground glass shadow with the progress of the disease, further lung consolidation | Focal B lines is the main feature in the early stage and in mild infection; alveolar interstitial syndrome is the main feature in the progressive stage and in critically ill patients; A lines can be found in the convalescence; pleural line thickening with uneven B lines can be seen in patients with pulmonary fibrosis |
Table 1: CT and ultrasonographic features of COVID-19 pneumonia [10].
Clinical case 2: Biopsy guided by ultrasound after CT- necrotic sample collection previously Figures 9A and 9B
A 66-year-old male was presented to the pulmonology department of Szeged University in Desk. A COPD patient with a history of choking (suffocation) and weakness was referred to chest CT to examine a lesion in the chest cavity. CT showed a round inhomogeneous soft tissue like on the right middle lobe, 9 cm in diameter, inseparable from hilus formulas. They are also found in the mediastinum and paratracheal. Laboratory findings showed a slightly increased WBC level. The patient was diagnosed with the trachea, bronchi, and lung tumors of uncertain or unknown behavior. The patient had a transthoracic needle biopsy (TNB) under CT-Guidance, but the sample that was collected did not obtain viable tissue; instead, necrotic tissue was collected. By using chest ultrasound as real-time guidance, we performed the second TNB procedure. Luckily, the tumor had margins with the chest wall pleura, making it easy to mark.
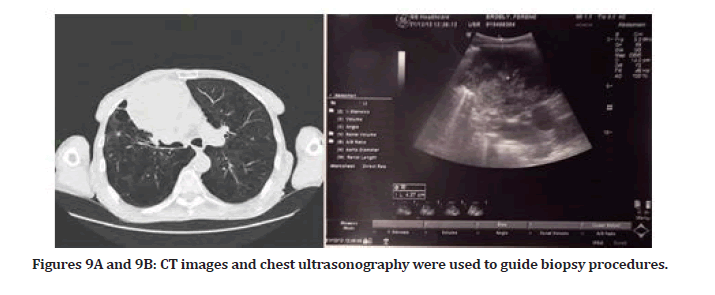
Figures 9A and 9B: CT images and chest ultrasonography were used to guide biopsy procedures.
CXR also showed 9 cm in the hilus.
A Head CT scan revealed that in the region of the temporal-parieto-occipitalis, a moderately hypodense band in the shape of a crescent with a maximum width of 13 mm was found. Subacute subdural hematoma is shown between the convexity of the brain parenchyma and the skull bones. Other intracerebral abnormalities were not visible. No fracture was observed on the skull, and no other intracranial volume-alternating lesion was shown.
Clinical case 3: Pleural effusion before diagnosis w/ bronchus or lung malignancy masses Figure 10A to 10F
On the right side of the chest, there are approximately 700 ml of liquid accumulation septum visible. An 8cm diameter, inhomogeneous soft tissue formula is shown. A puncture helix is inserted. When the patient was admitted, a chest CT with native plus iv contrast was ordered, which confirmed the presence of pleural effusion.
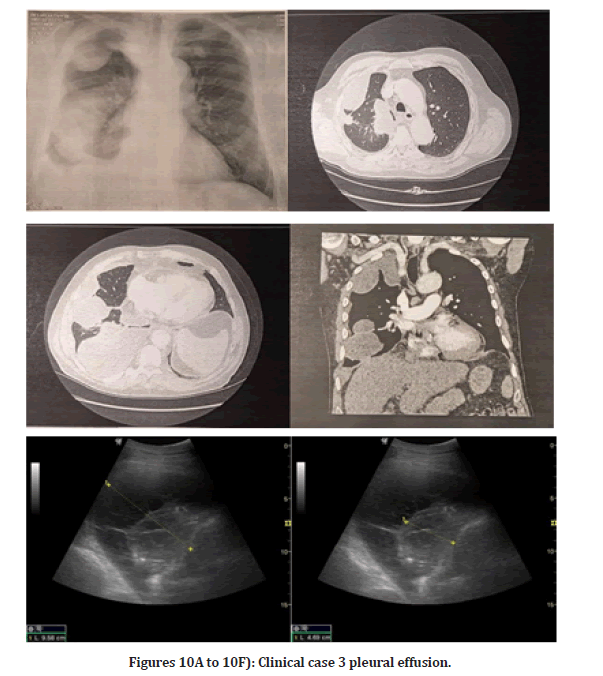
Figures 10A to 10F: Clinical case 3 pleural effusion.
History/Status: 70-year-old male complains of rightsided chest pain that radiates to his shoulder. CXR shows a decrease in the transparency of the right hemithorax. Angiography in the truncus pulmonary was performed, which showed no evidence of pulmonary embolism. On the right side a moderate amount of encapsulated liquid accumulation. COVID-19 pneumonia is commonly seen in intrapulmonary abnormalities not confirmed. The patient has mild mediastinal lymphadenomegaly and moderate cardiomegaly.
It is included by prior arrangement due to leaking fluid germs on the right. On CT of the thorax, a moderate amount of accumulated fluid was collected in the right thorax. On the next day of the chest CT finding, a chest puncture was performed following a chest ultrasound examination, during which 150 ml of chest fluid was aspirated for cytology, chemistry, microbiology, and B+R testing. Right pleuritis was confirmed. The course and width of the aorta are regular. Abdominal scans show no gross abnormalities. No abnormalities suggestive of bone malignancy have been identified.
The Conclusion of The Cases
The COVID-19 pneumonia patient demanded at least nine x-rays and a couple of CT scans during his stay in the intensive care unit, thus increasing his radiation exposure risk, despite several studies demonstrating the benefits of chest ultrasonography. Necrotic CT- necrotic sample collection: necrotic TNB tissue samples were collected under CT guidance, but there was no viable tissue extracted from the 66-year-old COPD patient who had previously complained of coughing and weakness. We successfully performed the second TNB procedure by utilizing chest ultrasonography with real-time guiding throughout the process to extract viable tissue. The 70-year-old gentleman arrives with pain spreading from his right chest to his shoulder. A chest CT was performed using native contrast and IV contrast to confirm the presence of pleural effusion. Using ultrasound guidance, 150 ccs of chest fluid was aspirated and subjected to cytology, chemical, and microbiological examinations. After a thorough analysis of ultrasound in general and chest ultrasound in particular, it is clear that it is an incredible imaging tool that would provide the simplest, most effective, and least expensive means of collecting and employing it with high accuracy, not to mention its role in aiding in a variety of guidance procedures, among other things. While chest X-rays are not as effective as ultrasonography for examining the chest organs and monitoring COVID-19 patients, the CT scan continues to establish its significance as the gold standard for imaging. Despite this, ultrasonography is accurate in diagnostic and guiding procedures when used in conjunction with CT.
Having said that, the use of chest US also has a few technical setbacks that clinicians may face. For example, clinicians may face issues in assessing obese patients using the US, mainly because of difficulties in visualizing certain structures due to the thickness of the subcutaneous skin and rib cage. Furthermore, sound wave transmission may be distorted in the setting of subcutaneous emphysema or if the patient has a large thoracic dressing. In addition, acoustic artifacts arising from subcutaneous emphysema or pleural calcifications may hinder the visualization of the pleural interface. Finally, it is important to note that usage expertise is required to be able to accurately assess and diagnose the findings, which thus prompts the need for a solid grasp of how it operates before proceeding with it.
Conclusion
While further studies are still needed to evaluate the importance of the role of chest US in the clinical setting, early data suggests that it may be superior to the use of CXR or CT in certain clinical scenarios. Its ability to accurately distinguish between the various acute respiratory illnesses in an emergency setting renders it an important tool in preventing the progression of time-sensitive diseases. Indeed, its ease of use and quick assessment time make it a desirable choice for many clinicians. Its properties have labeled it as one of the most revolutionary innovations in acute care medicine, particularly in our current epidemiological situation.
References
- Karthika M, Wong D, Nair SG, et al. Lung ultrasound: The emerging role of respiratory therapists. Respir Care 2019; 64:217-229.
- Lichtenstein DA. BLUE-protocol and FALLS-protocol: Two applications of lung ultrasound in the critically ill. Chest 2015; 147:1659-1670.
- Marini TJ, Weis JM, Baran TM, et al. Lung ultrasound volume sweep imaging for respiratory illness: a new horizon in expanding imaging access. Respir Res 2021; 8:e000919.
- Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet 2013; 381:1405-1416.
- Tsai TH, Jerng JS, Yang PC. Clinical applications of transthoracic ultrasound in chest medicine. J Med Ultrasound 2008; 16:7-25.
- Koh DM, Burke S, Davies N, et al. Transthoracic US of the chest: clinical uses and applications. Radiographics 2002; 22:e1.   Â
- Jambrik Z, Monti S, Coppola V, et al. Usefulness of ultrasound lung comets as a nonradiologic sign of extravascular lung water. Am J Cardiol 2004; 93:1265-1270.
- Hiorns MP, Screaton NJ, Müller NL. Acute lung disease in the immunocompromised host. Radiol Clin 2001; 39:1137-1151.
- Havelock T, Teoh R, Laws D, Gleeson F. Pleural procedures and thoracic ultrasound: British thoracic society pleural disease guideline 2010. Thorax 2010; 65:61-76.
- Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non-critical. Novel Coronavirus Pneumonia (COVID-19) SSRN. 2020; 3544750.
- Smith MJ, Hayward SA, Innes SM, et al. Pointâ?ofâ?care lung ultrasound in patients with COVIDâ?19: A narrative review. Anaesthesia 2020; 75:1096-1104.
- Tung-Chen Y. Lung ultrasound in the monitoring of COVID-19 infection. Clin Med 2020; 20:e62.
- Â Peng QY, Wang XT, Zhang LN. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019â??2020 epidemic. Intensive Care Med 2020; 46:849-850.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ali Abdullah Alnakhli1*, Fatimah Taher Alnakhli2 and Maryam Abdullah Alnakhli2
1EMS, Paramedic and MD, Szeged University, Szeged, Hungary2Medical Intern, MBBS, Taibah University, Madinah, KSA
Received: 14-Oct-2022, Manuscript No. jrmds-22-79673; , Pre QC No. jrmds-22-79673(PQ); Editor assigned: 17-Oct-2022, Pre QC No. jrmds-22-79673(PQ); Reviewed: 02-Nov-2022, QC No. jrmds-22-79673(Q); Revised: 01-Nov-2022, Manuscript No. jrmds-22-79673(R); Published: 08-Nov-2022
