Research - (2022) Volume 10, Issue 7
Clinicopathologic Characteristics of Ameloblastoma with Immunohistochemical Study
Maisa O Al-Sebaei1*, Soulafa A Almazrooa2, Sara K Akeel2, Ali S Sawan3 and Wafaey M Gomaa3,4
*Correspondence: Maisa O Al-Sebaei, Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, King Abdulaziz University, Saudi Arabia, Email:
Abstract
Introduction: Ameloblastoma is the most common benign odontogenic tumor that exhibits aggressive behavior. Aims: This study aimed to characterize and investigate the relationship between the immunostaining levels of multiple tumor markers and the clinical behavior of ameloblastoma. Materials and methods: All cases diagnosed with ameloblastoma were retrieved from the archives of the Department of Pathology. The clinical data were recorded along with histopathological characteristics. Immunohistochemistry was performed using the antibodies syndecan-1, p53, Ki67, and calretinin. Chi-square test, Kruskal–Wallis test, Wilcoxon signed-rank test were performed. Results and discussion: There were 25 cases of ameloblastoma and 10 control cases. The mean age was 32 years, and the male-to-female ratio was 1.2:1. The mandible was the most affected site (84%). The follicular pattern was the most seen variant. Bone invasion was seen in 5 cases. Ki67 immunostaining had no correlation with clinicopathologic parameters (tumor site, histologic variants, bone invasion, surgical resection margin, and recurrence). On the contrary, high syndican-1 immunostaining was associated with the follicular variant of ameloblastoma. Conclusion: The demographic data were consistent with the data reported in the literature. The Immunohistochemical staining pattern was consistent with that reported in the literature, but there was no significant correlation with the clinicopathologic parameters.
Keywords
Ameloblastoma, Immunohistochemistry, Recurrence, Odontogenic tumors, Retrospective studies
Introduction
Ameloblastoma is a benign, yet locally aggressive and infiltrative, odontogenic tumor that affects the bones of the mandible and maxilla. It is derived from epithelial odontogenic tissue, which is a part of the developing tooth germ. It can also be derived from the epithelial lining of odontogenic cysts. It is considered the most common odontogenic tumor in the maxillofacial region (9%–18%) [1].
The demographic data of patients affected by ameloblastoma are different from one region to another. Typically, ameloblastoma occurs more frequently in middle-aged individuals between the 3rd and 5th decade of life [1]. Previous studies reported 15.2% occurrence in children and adolescents [2]. There is no significant difference between males and females, with a ratio of 1.0–1.3:1.0 ratio [1,3]. Ameloblastoma can be asymptomatic, as it develops slowly in the mandible or maxilla, but the most common presenting symptoms are swelling and pain [1,4,5]. Radiographically, it presents as multilocular (soap-bubble appearance) or unilocular radiolucent lesions [2].
Traditionally, ameloblastoma has been classified into unicystic, solid (multicystic), and peripheral [1,6,7]. Peripheral ameloblastoma has an indolent behavior and needs conservative treatment in contrast to the other types of ameloblastoma. The unicystic type affects a younger population and is classified into luminal, intraluminal, and mural [8]. The solid type has more aggressive behavior and a high recurrence rate.
Diagnosis is established by microscopic examination. It exhibits distinct histologic features: epithelial islands that are arranged in follicles composed of basal cells with palisaded hyper chromatic nuclei with reversed polarity and sub nuclear vacuolization and inner stellate reticulum-like (follicular-type) cells or interconnected sheets of epithelium that present the same features but in a less prominent (plexiform-type) manner. Other histopathological subtypes can be seen, such as basal, acanthomatous, granular, and desmoplastic. The desmoplastic variant has slightly different clinical, biologic, and histopathological features than the others [7].
Definitive treatment of ameloblastoma comprises surgical resection of the affected bone with a 1-cm safety margin. Enucleation and curettage are not successful as an effective and definitive treatment modality, except in the case of unicystic ameloblastoma. Radical surgical intervention would decrease the recurrence rate from 52.0% to 6.1%, as shown by Milman et al [4].
The molecular and genetic profiling of ameloblastoma has been studied extensively in the literature. However, the mechanism and genotyping remain poorly understood. Tumor markers have been studied using immunohistochemistry to correlate these markers with tumor aggressiveness, behavior, prognosis, recurrence, and clinical outcome. The main tumor markers for ameloblastoma that were studied include CD138 (syndecan-1), p53, Ki67, and calretinin [9-15].
The decrease and absence of syndecan-1 was associated with more aggressive behavior and poor clinical outcomes [11]. p53 expression was also observed to be overexpressed in ameloblastoma compared to other odontogenic tumors, which indicates more aggressive behavior [12]. Ki67 expression was lower than 10%, [14] although a study by Abdel-Aziz et al showed a significant association between a high percentage of Ki67 and recurrence [13]. Calretinin was also demonstrated to be a useful Immunohistochemical marker for ameloblastoma as an adjunct method to differentiate it from other odontogenic cysts if confusion occurs [9,10].
The current study aims to establish a database of patients diagnosed with ameloblastoma at King Abdulaziz University Hospital, Jeddah, Saudi Arabia. Following this, it aims to identify the clinical characteristics of ameloblastoma unique to the Saudi Arabian population through retrospective and prospective profiling of the affected patients in terms of race, age, gender, and habits. In addition, it aims to identify the specific histologic subtypes of ameloblastoma more common in our region and identify and quantify specific tumor markers through immunohistochemistry, which can be correlated with tumor aggressiveness, prognosis, recurrence, and clinical outcome in resected ameloblastoma.
Materials and Methods
A retrospective review of 25 patients diagnosed with ameloblastoma at the Department of Pathology and the Division of Oral and Maxillofacial Pathology in the Faculty of Dentistry (both at King Abdulaziz University Hospital) was conducted from January 2005 to December 2016. The clinicopathologic characteristics of the patients were collected from patient records. Hematoxylin and eosin slides of these patients were reviewed. Paraffin wax blocks were retrieved from the archives of the department. In addition, 5 cases each of dentigerous cysts and odontogenic keratocysts were collected to be used as the control group. The study was approved by the Research Committee of the Biomedical Ethics Unit, Faculty of Medicine, King Abdulaziz University (no 255-20).
The paraffin wax blocks of ameloblastoma were cut at 4 μm and mounted on positively charged slides (Leica Microsystems Plus Slides). The sections were deparaffinized in xylene and rehydrated in an automated immunostainer (BenchMark XT; Ventana Medical Systems, Tucson, AZ, USA). Pretreatment was performed using CC1 (a prediluted cell conditioning solution) for 60 minutes. Primary antibodies were applied as follows (Table 1): Ki67 (SP6) rabbit monoclonal antibody (Cell Marque, South Holland, Netherlands), monoclonal mouse antihuman CD138 antibody (DakoCytomation, Norden, Glostrup, Denmark), monoclonal mouse antihuman p53 antibody (DakoCytomation, Norden, Glostrup, Denmark), and rabbit monoclonal antihuman calretinin antibody (Cell Marque, South Holland, Netherlands). The antibodies were incubated at 37°C for 20 minutes. A Ventana iVIEW DAB detection kit was used according to the manufacturer’s instructions. Subsequently, the slides were washed, counterstained with Mayer’s hematoxylin, and mounted. The negative (substitution of primary antibody with Tris-buffered saline) and positive control slides were included as appropriate for each antibody.
| Antibody | Clone | Company | Dilution |
|---|---|---|---|
| p53 | D0.7 | Dako Cytomation | 1:25 |
| Ki67 | MIB1 | Dako Cytomation | 0.09375 |
| Calretinin | SP13 | Cell Marque | 0.180555556 |
| CD138 | MI15 | Dako Cytomation | 1:25 |
Table 1: A summary of the primary antibodies used and their dilution.
The immunohistochemical slides were interpreted by anatomic and oral and maxillofacial pathologists. Immunostaining of the antibodies was expressed as the percentage of positive tumor (or squamous) cells/ the total number of tumor cells. The percentage of positive cells was used to classify immunostaining as follows: negative—<5% of the cells positive (score 1); low immunostaining—5%–50% of the cells positive (score 2); and high immunostaining—>50% of the cells positive (score 3).
A one-sample chi-square test was conducted to compare the different categories of 1 antibody. The Kruskal– Wallis test was used to detect the differences between more than 2 groups. The Wilcoxon signed-rank test was performed to compare 2 related samples. The statistical procedures were performed using SPSS for Windows (v16.0.; SPSS, Chicago, IL, USA). Statistical significance was determined at a p¬-value of ≤0.05 and was 2-sided.
Results
A total of 25 cases were diagnosed with ameloblastoma. The mean age of the patients was 32 (range: 12–79) years. There were a male:female ratio of 1.2:1.0. The mandible was more commonly affected (84%). Radiographically, the majority of the lesions presented as multilocular radiolucent lesions in the mandible (72%). Clinically, they appeared with variable degrees of swelling and expansion. Some large lesions involved tooth mobility and pain (Table 2). There were 4 cases with cystic ameloblastoma, 2 of which showed mural invasion. The most common histopathological type was the follicular variant (60%). Bone invasion was present in 5 cases (20%). The surgical resection margins were free in 15 (60%) cases. Local recurrence was found in 2 (8%) cases. For the control group, 10 cases of dentigerous cysts (n=5) and odontogenic keratocysts (n=5) were included. Data are presented in (Table 2), and the histologic examples of ameloblastoma and control cases are shown in (Figure 1).
| Number (%) | ||
|---|---|---|
| Sex | Males | 16 (53.8%) |
| Females | 9 (46.1%) | |
| Tumor site | Mandible | 21 (84.0%) |
| Maxilla | 4 (16.0%) | |
| Radiographic presentation | Unilocular | 4 (16.0%) |
| Multilocular | 18 (72.0%) | |
| Clinical presentation | Swelling | 19 (76.0%) |
| Tooth mobility | 2 (8.0%) | |
| Pain | 3 (12.0%) | |
| Histologic type | Follicular | 15 (60.0%) |
| Plexiform | 2 (8.0%) | |
| Acanthomatous | 2 (8.0%) | |
| Granular | 1 (4.0%) | |
| Desmoplastic | 1 (4.0%) | |
| Cystic | 4 (16.0%) | |
| Bone invasion | Absent | 20 (80.0%) |
| Present | 5 (20.0%) | |
| Surgical resection margin | Free | 15 (60.0%) |
| Involved | 2 (8.0%) | |
| Not known | 8 (32.0%) | |
| Recurrence | Absent | 23 (92.0%) |
| Present | 2 (8.0%) | |
Table 2: Clinicopathologic features of ameloblastoma cases (n=25).
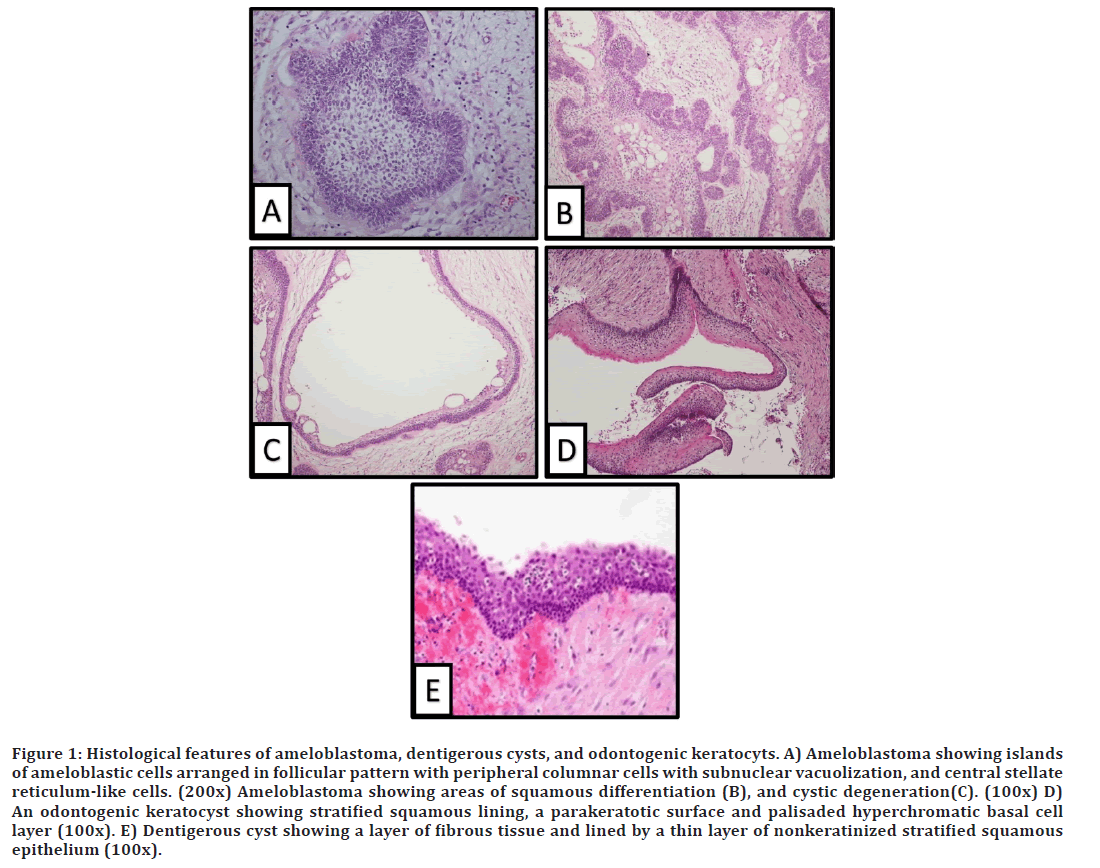
Figure 1. Histological features of ameloblastoma, dentigerous cysts, and odontogenic keratocyts. A) Ameloblastoma showing islands of ameloblastic cells arranged in follicular pattern with peripheral columnar cells with subnuclear vacuolization, and central stellate reticulum-like cells. (200x) Ameloblastoma showing areas of squamous differentiation (B), and cystic degeneration(C). (100x) D) An odontogenic keratocyst showing stratified squamous lining, a parakeratotic surface and palisaded hyperchromatic basal cell layer (100x). E) Dentigerous cyst showing a layer of fibrous tissue and lined by a thin layer of nonkeratinized stratified squamous epithelium (100x).
In ameloblastoma cases, nuclear Ki67 showed strong positivity nuclear staining in basal and stellate central cells, and p53 nuclear staining was observed as being scattered in basal and stellate central cells. Membranous syndecan-1 (CD138) immunostaining was more observed in the central portion of follicular cells than in basal cells. Also, there was positive staining in the stroma. Calretinin exhibited weak nuclear and cytoplasmic staining predominately in most basal cells. Negative Ki67 immunostaining was statistically significantly more than lower and higher immunostaining (p=0.009). Syndecan-1 (CD138) was detected in all cases; however, there was no difference between low and high immunostaining. For p53, there was no high immunostaining, and many cases did not express p53, but there was no difference between negative and low immunostaining. Calretinin immunostaining—neither cytoplasmic nor nuclear—was negative in the majority of cases (p=0.072 and 0.009). Data are provided in (Table 3), and representative examples are shown in (Figure 2).
| Negative n (%) | Low immunostaining n (%) | High immunostaining n (%) | p-value* | |
|---|---|---|---|---|
| Ki67 | 19 (76%) | 6 (24%) | 0 (0%) | 0 |
| Syndecan-1 (CD138) | 0 (0%) | 9 (36%) | 16 (64%) | 0.162 |
| p53 | 13 (52%) | 12 (48%) | 0 (0%) | 0.841 |
| Calretinin cytoplasmic | 17 (68%) | 8 (32%) | 0 (0%) | 0.072 |
| Calretinin nuclear | 19 (76%) | 6 (24%) | 0 (0%) | 0.009 |
| * 1-sample chi-square test. | ||||
Table 3: Immunostaining of antibodies in ameloblastoma cases (n=25).
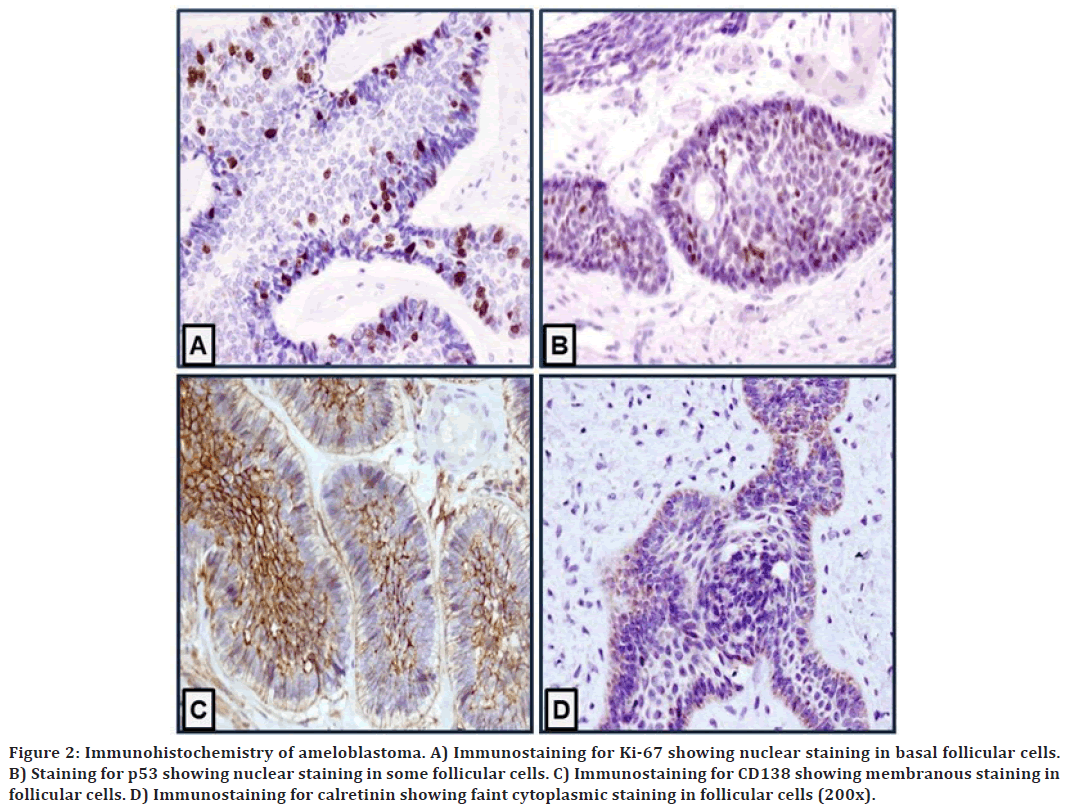
Figure 2. Immunohistochemistry of ameloblastoma. A) Immunostaining for Ki-67 showing nuclear staining in basal follicular cells. B) Staining for p53 showing nuclear staining in some follicular cells. C) Immunostaining for CD138 showing membranous staining in follicular cells. D) Immunostaining for calretinin showing faint cytoplasmic staining in follicular cells (200x).
In the control cases, Ki67 showed a uniform basal squamous epithelial layer and strong nuclear immunostaining. For CD138, cysts displayed strong membranous immunostaining in all the layers of squamous epithelium. Calretinin showed negative immunostaining in squamous epithelium in all cases. On the contrary, p53 exhibited patchy staining with weak to moderate staining intensity in the squamous epithelium in all cases. Data are provided in Table 4, and representative examples are shown in (Figure 3).
| Negative, n (%) | Low immunostaining, n (%) | High immunostaining, n (%) | p-value* | |
|---|---|---|---|---|
| Ki67 | 9 (90%) | 1 (10%) | 0% | 0.011 |
| Syndecan-1 (CD138) | 0% | 1 (10%) | 9 (90%) | 0.011 |
| p53 | 2 (20%) | 8 (80%) | 0 (0%) | 0.05 |
| Calretinin cytoplasmic | 10 (100%) | 0% | 0% | 0.001 |
| Calretinin nuclear | 10 (100%) | 0% | 0% | 0.001 |
| * 1-sample chi-square test. | ||||
Table 4: Immunostaining of antibodies in control cases (n=10).
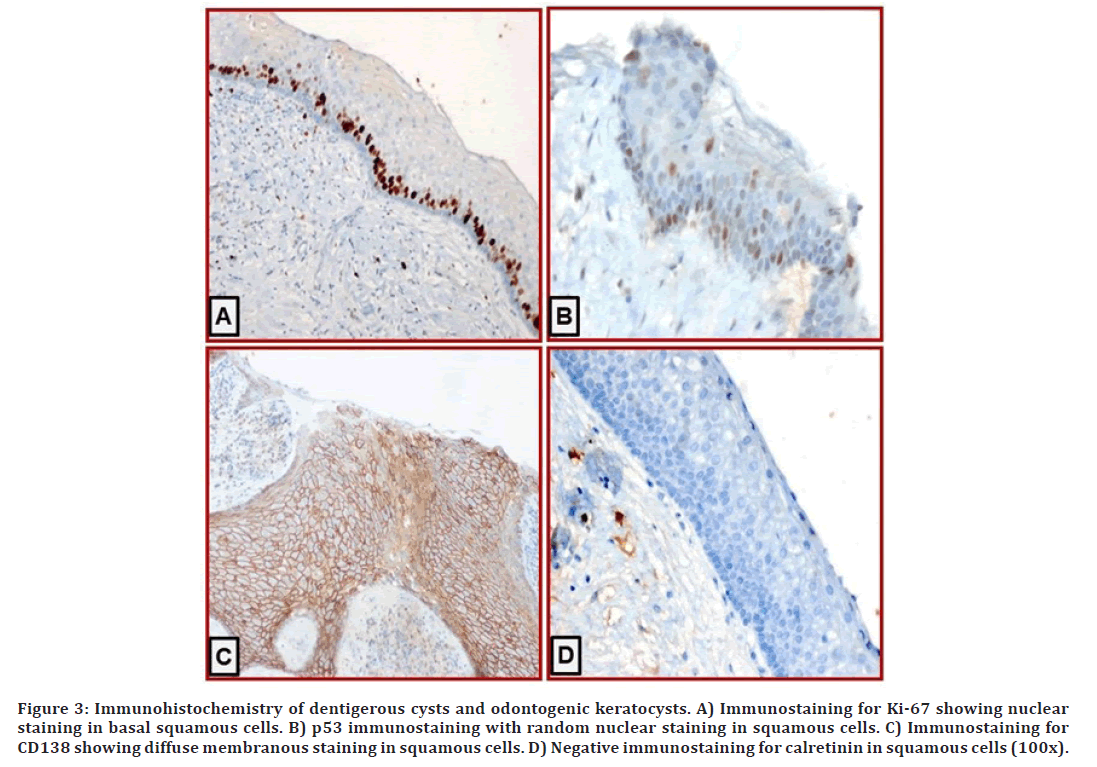
Figure 3. Immunohistochemistry of dentigerous cysts and odontogenic keratocysts. A) Immunostaining for Ki-67 showing nuclear staining in basal squamous cells. B) p53 immunostaining with random nuclear staining in squamous cells. C) Immunostaining for CD138 showing diffuse membranous staining in squamous cells. D) Negative immunostaining for calretinin in squamous cells (100x).
There was no statistically significant difference in immunostaining between ameloblastoma and control cases for Ki67 (p=0.738), CD138 (p=0.211), p53 (p=0.453), calretinin cytoplasmic (p=0.981), or calretinin nuclear (p=0.945).
In ameloblastoma cases, syndecan-1 (CD138) showed more cases with high immunostaining than did Ki67 (p=0.008), p53 (p>0.001), calretinin cytoplasmic (p>0.001), and calretinin nuclear (p>0.001) immunostaining. Also, there was no statistically significant difference in immunostaining between Ki67, p53, and calretinin (cytoplasmic and nuclear).
Ki67 immunostaining showed no correlation with clinicopathologic parameters (tumor site, histologic variants, bone invasion, surgical resection margin, and recurrence). In contrast, high syndican-1 (CD138) immunostaining was associated with the follicular variant of ameloblastoma (p=0.02). Plexiform ameloblastoma showed low expression compared to the follicular variant, which showed negative expression. Other antibodies exhibited no correlation with clinicopathologic features (Table 5).
| Sex | Tumor site | Histologic variants | Bone invasion | SRM | Recurrence | |
|---|---|---|---|---|---|---|
| Ki67 | 0.878 | 0.193 | 0.746 | 0.819 | 0.557 | 0.379 |
| Syndecan-1 (CD138) | 0.05 | 0.624 | 0.047 | 0.221 | 0.117 | 0.673 |
| p53 | 0.17 | 0.026 | 0.166 | 0.17 | 0.925 | 0.954 |
| Calretinin cytoplasmic | 0.327 | 0.049 | 0.991 | 0.529 | 0.029 | 0.035 |
| Calretinin nuclear | 0.878 | 0.96 | 0.614 | 0.819 | 0.187 | 0.379 |
| SRM, surgical resection margin. | ||||||
| * The Kruskal–Wallis test was used. (The numbers represent the p-value.) | ||||||
Table 5: Association of different types of immunostaining with clinicopathologic features of ameloblastoma cases*.
Discussion
Ameloblastoma is a benign locally aggressive odontogenic tumor that can affect the maxilla and mandible. It is derived from odontogenic epithelium. There was a somatic mutation affecting the cell proliferation control mitogen-activated protein kinase signaling pathway [16]. BRAF mutation was more frequently seen in mandibular tumors, whereas the sonic hedgehog pathway— specifically activating mutations in Smoothened— was seen in maxillary tumors [17-19]. It is treated aggressively to decrease the chance of recurrence [16]. There were limited data in the literature discussing the clinicopathologic features of ameloblastoma in Saudi Arabia. This study was conducted to compare the results of our center with those of the rest of the world.
Reichart et al reviewed and reported the largest series of ameloblastoma in 3677 cases in 33 years [1]. Saghravanian et al reported 88 cases in 40 years in Iran, whereas Milman reported 54 cases in 25 years in the USA [4,5]. Sing reported 42 cases in 11 years in Australia [20]. The mean age of patients in these studies ranged from 30 to 53 years. In the current study, the mean age was within this range (32 years), with a male-to-female ratio of 1.2:1.0, which is similar to what was reported in the literature that showed an equal ratio or slight male predilection.
Ameloblastoma can also occur at a young age in those as young as 3 years old [3]. Bansal et al reported their 41 years of experience with pediatric patients and found 39 cases (15.2% of all ameloblastoma cases) [2]. Their ages ranged between 4.5 and 18 years, with a male-to-female ratio of 2:1. More than 90% of the cases presented in the mandible. Almost half were conventional ameloblastoma cases, and the other half were unicystic ameloblastoma cases [2]. In the current study, 7 affected cases included children aged 2–18 years. Five of these cases were boys and 2 were girls, with a ratio of 2.5:1.0. It was found to affect the mandible in all the boys and the maxilla in the girls. Two of these cases were unicystic ameloblastoma, and the rest were conventional ameloblastoma cases. It commonly affects African Americans, but in this series, racial information was not available, as it was not one of the mandatory data to be collected.
In the literature, the mandible was the most common location, and the most common presenting sign was swelling in the affected jaw, which is similar to the current study. The most common histopathologic variant reported in this study and the literature was the follicular variant; furthermore, the follicular and plexiform variants can coexist or any other 2 variants together [4]. Studies have shown that there is no correlation between histopathologic type and clinical behavior [21]. Other reports showed that follicular ameloblastoma had the highest recurrence rate [22].
The mainstay of treatment for ameloblastoma is surgical excision [16]. The most common surgical approach is wide local excision with proper margins and immediate reconstruction [16]. Multiple studies have indicated that the initial surgical intervention is significantly correlated with recurrence [23,24]. In the current study, all cases were treated by surgical resection, with a margin of 1 cm. Those with free surgical margins did not recur, whereas those with positive margins showed recurrence.
In the literature, there are studies discussing the stain of certain Immunohistochemical markers in ameloblastoma. The purpose of these studies varies in the context of whether to differentiate diseases from each other or to check the behavior of the diseases, which would influence the treatment and prognosis. Previous studies showed a low proliferation index (Ki67), and if high, it was associated with high recurrence, but that did not show significant results in the current study [13-15].
In addition, the literature showed that p53 exhibited stronger expression in plexiform ameloblastoma and more at the periphery of the cells [12]. Kramer et al reported that in addition to the strong expression in the plexiform variant, the follicular variant—specifically acanthomatous and granular—did not express p53, whereas the basal and desmoplastic variants showed scattered reactivity. p53 is a tumor suppressor gene that indicates proliferation and a greater tendency toward malignant transformation. The current study results indicate low immunostaining in less than half of the scattered cells (Table 3). Of these cases, plexiform and combined plexiform/follicular ameloblastoma were the positive cases, which is consistent with what has been published in the literature.
Calretinin is another marker that is used for ameloblastoma. It is naturally expressed in neural tissues [9]. It was demonstrated to be present and positive in some neoplastic processes. Past studies reported its unique expression in ameloblastoma in comparison to other odontogenic cysts [9, 25]. This can be helpful specifically in unicystic ameloblastoma, which can sometimes be challenging. Altini et al and Coleman et al reported strong positive staining in stellate reticulum-like cells and most superficial layers of the cells in unicystic ameloblastoma and the central part of ameloblastic islands in conventional ameloblastoma [10]. In the current study, cases showed negative or low immunostaining (Table 3) in comparison to other studies. Does that suggest the stain is not reliable, as claimed by past studies?
Syndecan-1 (CD138) is a heparan sulfate proteoglycan that is mainly present in the stroma of ameloblastoma and has also been reported in stellate reticulum-like cells It promotes cell adhesion to the extracellular matrix, which holds cells in place so that they cannot migrate [26]. It is a prognostic marker if the expression is lost, which indicates poor clinical outcome [26]. Alotaibi et al reported the presence of syndecan-1 in ameloblastoma, odontogenic keratocysts, and dentigerous cysts. The expression in ameloblastoma is much lower than that in the others, which indicates higher proliferative activity [11]. In the current study, syndecan-1 (CD 138) was present in all cases in variable expression. (Half showed high immunostaining and the other half low immunostaining.) It was present in the stellate reticulum as well as the stroma. On the basis of the previous study results, does that mean half of our cases (with low immunostaining) were more aggressive than the other half (with high immunostaining)? Ameloblastic carcinoma cases exhibited high syndecan-1 (CD138) expression.
Bologna-Molina et al. reported lower expression of Ki67 in desmoplastic ameloblastoma and high expression in ameloblastic carcinoma [27]. Safadi et al found a significant difference between the expression in ameloblastoma—whether recurrent or not—and ameloblastic carcinoma, which had higher expression [28]. In the current study, Ki67 was low or lost in all cases. This is consistent with the results for the p53 marker. Both Ki67 and p53 indicated more proliferative activity.
In the current study, all the aforementioned Immunohistochemical stains did not correlate significantly with any of the Clinicopathologic parameters that were measured (i.e., tumor site, histologic variant, bone invasion, surgical resection margin, and recurrence), except for syndecan-1 with the follicular variant. There was a statistically significant association between the strength of syndican-1 expression and follicular ameloblastoma, which is interpreted as good clinical behavior. This is a surprising result of Ki67 and p53 in ameloblastic carcinoma, which is expected to be high, but it did not show that. The lack of a significant association in the previous results can be attributed to several reasons. This can be attributed to the low number of cases and the short duration of the followup period. Technique-sensitive procedures and antigen retrieval mechanisms can also influence this.
Conclusion
p>The demographic data gathered from the patients diagnosed with ameloblastoma were in alignment with the data reported in the literature. The staining pattern of Ki67, syndecan-1 (CD138), p53, and calretinin was consistent with that reported in the literature, but there was no significant correlation with the clinicopathologic parameters examined (such as histologic variant, bone invasion, surgical resection margin, and recurrence), except syndican-1 with follicular ameloblastoma, indicating good clinical behavior.Funding
The project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, under grant number (013/9/1429).
References
- Reichart PA, Philipsen HP, Sonner S. Ameloblastoma: Biological profile of 3677 cases. Eur J Cancer Part B. 1995; 31:86-99.
- Bansal S, Desai RS, Shirsat P, et al. The occurrence and pattern of ameloblastoma in children and adolescents: an Indian institutional study of 41 years and review of the literature. Int J Oral Maxillofac Surg 2015: 44:725-731.
- Chaudhary Z, Krishnan S, Sharma P, et al. A review of literature on ameloblastoma in children and adolescents and a rare case report of ameloblastoma in a 3-year-old child. Craniomaxillofac Trauma Reconstr 2012: 5:161-168.
- Milman T, Ying GS, Pan W, et al. Ameloblastoma: 25 Year experience at a single institution. Head Neck Pathol 2016; 10:513-520.
- Saghravanian N, Salehinejad J, Ghazi N, Shirdel M, Razi M. A 40-year retrospective clinicopathological study of ameloblastoma in Iran. Asian Pacific Journal of Cancer Prevention. 2016;17(2):619-23.
- Philipsen HP, Reichart PA, Nikai H, et al. Peripheral ameloblastoma: Biological profile based on 160 cases from the literature. Oral Oncol 2001; 37:17-27.
- Philipsen HP, Reichart PA, Takata T. Desmoplastic ameloblastoma (including "hybrid" lesion of ameloblastoma). Biological profile based on 100 cases from the literature and own files. Oral Oncol 2001; 37:455-460.
- Philipsen HP, Reichart PA. Unicystic ameloblastoma. A review of 193 cases from the literature. Oral Oncol 1998; 34:317-325.
- Anandani C, Metgud R, Singh K. Calretinin as a diagnostic adjunct for ameloblastoma. Pathol Res Int 2014; 2014:308240.
- Altini M, Coleman H, Doglioni C, et al. Calretinin expression in ameloblastomas. Histopathol 2000; 37:27-32.
- Al-Otaibi O, Khounganian R, Anil S, et al. Syndecan-1 (CD 138) surface expression marks cell type and differentiation in ameloblastoma, keratocystic odontogenic tumor, and dentigerous cyst. J Oral Pathol Med 2013; 42:186-193.
- Barboza CA, Pereira Pinto L, Freitas Rde A, et al. Proliferating cell nuclear antigen (PCNA) and p53 protein expression in ameloblastoma and adenomatoid odontogenic tumor. Braz Dent J 2005; 16:56-61.
- Abdel-Aziz A, Amin MM. EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: possible role in local recurrence. Diagn Pathol 2012; 7:14.
- Florescu A, Simionescu C, Ciurea R, et al. P53, Bcl-2 and Ki67 immunoexpression in follicular solid ameloblastomas. Rom J Morphol Embryol 2012; 53:105-109.
- Olimid DA, Florescu AM, Cernea D, et al. The evaluation of p16 and Ki67 immunoexpression in ameloblastomas. Rom J Morphol Embryol 2014; 55:363-367.
- McClary AC, West RB, McClary AC, et al. Ameloblastoma: A clinical review and trends in management. Eur Arch Otorhinol 2016; 273:1649-1661.
- Kurppa KJ, Caton J, Morgan PR, et al. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol 2014; 232:492-498.
- Sweeney RT, McClary AC, Myers BR, et al. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet 2014; 46:722-725.
- Mishra P, Panda A, Bandyopadhyay A, et al. Sonic Hedgehog Signalling Pathway and Ameloblastoma-A review. J Clin Diagnostic Res 2015; 9:ZE10.
- Singh T, Wiesenfeld D, Clement J, et al. Ameloblastoma: Demographic data and treatment outcomes from Melbourne, Australia. Aust Dent J 2015; 60:24-29.
- Gardner DG. Some current concepts on the pathology of ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996; 82:660-669.
- Ueno S, Mushimoto K, Shirasu R. Prognostic evaluation of ameloblastoma based on histologic and radiographic typing. J Oral Maxillofac Surg 1989; 47:11-15.
- Hong J, Yun PY, Chung IH, et al. Long-term follow up on recurrence of 305 ameloblastoma cases. Int J Oral Maxillofac Surg 2007; 36:283-288.
- Almeida Rde A, Andrade ES, Barbalho JC, et al. Recurrence rate following treatment for primary multicystic ameloblastoma: Systematic review and meta-analysis. Int J Oral Maxillofac Surg 2016; 45:359-367.
- D'Silva S, Sumathi MK, Balaji N, et al. Evaluation of calretinin expression in ameloblastoma and non-neoplastic odontogenic cysts-An immunohistochemical study. J Int Oral Health 2013; 5:42-48.
- Leocata P, Villari D, Fazzari C, et al. Syndecan-1 and wingless-type protein-1 in human ameloblastomas. J Oral Pathol Med 2007; 36:394-399.
- Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N, et al. Comparison of the value of PCNA and Ki-67 as markers of cell proliferation in ameloblastic tumors. Med Oral, Patol Oral Cir Bucal 2013; 18:e174.
- Safadi RA, Quda BF, Hammad HM. Immunohistochemical expression of K6, K8, K16, K17, K19, maspin, syndecan-1 (CD138), alpha-SMA, and Ki-67 in ameloblastoma and ameloblastic carcinoma: Diagnostic and prognostic correlations. Oral Surg Oral Med Oral Pathol Oral Radiol 2016; 121:402-411.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Maisa O Al-Sebaei1*, Soulafa A Almazrooa2, Sara K Akeel2, Ali S Sawan3 and Wafaey M Gomaa3,4
1Department of Oral and Maxillofacial Surgery, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia2Department of Oral Diagnostic Sciences, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia
3Department of Pathology, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
4Department of Pathology, Faculty of Medicine, Minia University, Al Minia, Egypt
Received: 02-Jul-2022, Manuscript No. JRMDS-22-68299; , Pre QC No. JRMDS-22-68299 (PQ); Editor assigned: 04-Jul-2022, Pre QC No. JRMDS-22-68299 (PQ); Reviewed: 19-Jul-2022, QC No. JRMDS-22-68299; Revised: 22-Jul-2022, Manuscript No. JRMDS-22-68299 (R); Published: 29-Jul-2022
