Research - (2022) Volume 10, Issue 9
Colorectal Cancer Diagnosis by detection of MicroRNA-92 in the Serum
Ali Hassan A Ali1,2*, Mohammed H Karrar Alsharif1, Mohammed Mobarak M Almudarra3, Turky Saad AlGraene3, Moataz Daadour3, Yousef M Alsomali3, Mohammed Abdullah Aldossari3 and Ahmed M Alsomali3
*Correspondence: Ali Hassan A Ali, Department of Anatomy, College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, KSA, Email:
Abstract
Colorectal cancer is the third most prevalent tumor worldwide, with a median age of 53 years and a predominance in men. We desperately require a gold standard for early detection and non-invasive diagnosis. Micro RNA 92a, a novel tumor marker, was shown to be highly concentrated in the serum of colorectal cancer patients. The present study aimed to assess the miR-92a expression level as a stable blood-based biomarker for the detection of colorectal cancer. This prospective observational study was carried out on 65 subjects. The cases group comprised 44 consecutive naive patients with colorectal cancer proved by colonoscopy and histopathological examination of biopsied specimens. Twenty-one subjects without colorectal cancer and normal colonoscopy served as the control group. Quantitative real-time RT-PCR was applied to determine the relative expression level of miRNA-92a in serum. According to the findings of the histopathological examination of surgically removed specimens, the final stage of colorectal cancer patients was determined. Serum miR-92a expression was significantly higher in the cases group compared to the control. Serum miR-92a levels revealed a significant positive correlation with both TNM (P=0.008) and MAColler stages (P=0.014), as well as a highly significant positive correlation with the tumor size (P=0.001). A statistically significant positive correlation was found between age (p= 0.005) and no significant Correlation between serum miR-92a level with sex and Duke's stage. Serum levels of miR-92a were significantly higher in the cases group. Serum levels of miR-92a showed a significant positive correlation with both TNM and MAColler stages.
Keywords
Colorectal cancer, Marker, Microrna-92a, Diagnosis
Introduction
Around the world, colorectal cancer (CRC) incidence and fatality rates vary significantly. With 1.4 million new cases and an anticipated 694,000 deaths in 2012, CRC is the second most frequent cancer in women and the third most common disease in men [1]. The decrease in CRC incidence and mortality rates over the past 20 years in the United States is largely related to screening's role in early detection and prevention [2]. Overall, men have a 30%–40% greater incidence and mortality rate from CRC than women. Although the causes are not fully understood, they probably result from the complicated interplay between risk factors and gender-related changes in hormone exposure [3]. With incidence rising steadily beyond age 50, aging is the main risk factor for CRC. People older than 50 are diagnosed with more than 90% of the cases [4].
A future colon or rectum cancer is more likely to occur in people with a personal history of CRC. Younger diagnostic age is linked to a higher risk [5]. There are currently few serum biomarkers with varying sensitivity and specificity levels utilized for screening or early detection of CRC [6]. Small non-coding RNAs known as microRNAs have a significant role in the control of cell differentiation, mitosis, apoptosis, and carcinogenesis [7,8]. Because they are more likely to be discovered at an earlier stage, tumors that present with rectal bleeding (usually those involving the distal colon and rectum) have been assumed to have a better prognosis [9]. Numerous cancers have been linked to miR-dysregulation in research conducted over the past ten years, and this number is continuously growing. Other cancers followed after the first description of miR-15a and -16-1 in B-cell chronic lymphocytic leukemia. Glioblastoma, breast cancer, acute myeloid leukemia, melanoma, pancreatic cancer, lung cancer, ovarian cancer, hepato-cellular carcinoma, thyroid cancer, bladder and kidney cancer, gastric cancer, colorectal cancer, and other cancers have all been linked to a variety of miR dysregulation [10].
The aim of our work is to assess the miR-92a (MicroRNA- 92a) expression level as a stable blood-based biomarker for the detection of colorectal cancer.
Subjects and Methods
This prospective observational study was carried out on 65 subjects. The cases group comprised 44 consecutive patients with CRC who were attending the Departments of General Surgery and Hepatology, Gastroenterology & Infectious Diseases at King Khalid Hospital, AlKharj, within the period between January 2022 and June 2022. The Ethical Committee approved the study protocol of Prince Sattam bin Abdulaziz University Institutional Review Board. (PSAU-2022 ANT 79/43PI).
21 additional seemingly healthy individuals without CRC and normal colonoscopies (The indication for colonoscopy in such healthy subjects was either a long history of abdominal pain, flatulence, and/or altered bowel habits) served as the control group. The studied cases group comprised 30 males and 14 females with a mean age of 51.5 ± 15.1 years. All had sporadic CRC, proved by colonoscopy and histopathology. Patients with the following criteria were excluded from the study: patients who refused the written consent, pregnant females, patients with familial adenomatous polyposis or hereditary nonpolyposis CRC, and those who received chemotherapy or radiotherapy. All the studied cases were subjected to informed written medical consent. Full history taking focusing on family history of CRC, history of chronic constipation, bleeding per rectum, significant weight loss, and/or anemia of unexplained etiology. Local general and PR examinations were performed, focusing on cachexia, pallor, and palpable abdominal lymphadenopathy, respectively.
Detection of microRNA- 92a expression level using realtime PCR technique Extraction of total RNA including microRNA-92a from plasma samples using microRNA extraction kit according to Wu, et al. [11]. Relative quantitation of microRNA-92a level using real-time quantitative PCR (RT-PCR) according to Huang, et al. [12]. Multiple biopsies were taken from any suspected lesion and were sent for histopathological examination. TNM, Duke's, and MACcoller staging Scores were applied. The Kolmogorov-Smirnove test, Student "t" if quantitative data were regularly distributed, or Man Whitney U test, Krauskal Wallis test, and Spearman's correlation coefficient (rho) if they were not. The optimal cutoff values for microRNA-92a were found using the ROC curve to predict colorectal cancer patients, as well as to identify particular T stage and size and MACcoller stage.
Results
The main presenting symptom in the studied cases was bleeding per rectum (22/44=50%) followed by chronic constipation (19/44=43.1%) and significant weight loss (10/44=22%). Positive family history was found in (11/44=25%) of the studied cases (Table 1 and Figure 1). The main clinical examination findings in the studied cases were pallor (29/44=65.9%), cachexia (18/44=40.9 %), palpable anorectal mass by PR examination (12/44=27.2 %) and lastly palpable abdominal mass (9/44=20.4 %).
| Variable | Cases (44) | Control (21) | P | |
|---|---|---|---|---|
| Age | 50.6 ± 15.1 | 54.1 ± 13.0 | 0.37 | |
| Sex | Male | 30 | 15 | 0.71 |
| Female | 14 | 7 | ||
Table 1: Age and sex distribution in the studied groups.
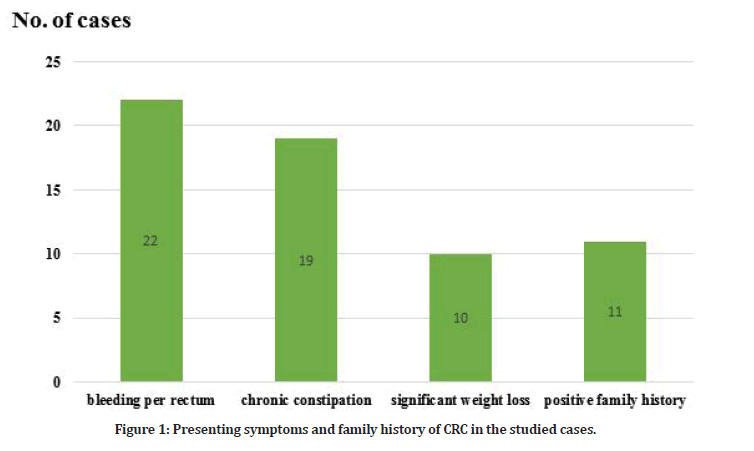
Figure 1: Presenting symptoms and family history of CRC in the studied cases.
Colonoscopy examination of the studied cases revealed that the main lesion site of CRC was the colon (29/44=65.9%), followed by the rectum (12/44=27.27%) and lastly recto sigmoid (5/44=11.3%). Small lesions (< 2.5 cm) were seen in 9 cases (20.4%), medium-sized ones (2 – 5 cm) were19 cases (43.1 %) , while large lesions were seen in 23 cases (51.4%). Mass was the main lesion seen; only three cases showed an ulcer, and another one showed a stricture.
The main histopathological CRC types in the studied cases were adenocarcinoma (30/44=68.1%) followed by Mucinous adenocarcinoma (11/44=25 %) and Signet ring cell adenocarcinoma (3/44=6.8%). The majority of the studied cases were Duke B (87.9%). According to TNM staging, cases with TIIB were (59.4%) followed by TIIA (36.2%). Three cases only were Tis (7.4%) (Tables 2 and 3, Figure 2). Serum miR-92a expression was significantly higher in the cases group compared to the control (6.03 ± 0.324 ≠ 5.65 ± 0.20 log RU (Table 4). There was a statistically highly significant positive correlation between the tumor size and serum microRNA-92a expression. TNM-stages (P=0.008) and MACcoller- stages (p=0.014) with serum microRNA 92a expression. Other assessed variables showed a nonsignificant correlation. A statistically highly significant positive correlation was found between serum MiR92a and tumor size in the studied cases.
| Type | No. of cases (44) | Percentages |
|---|---|---|
| Adenocarcinoma | 30 | 68.1 |
| Mucinous adenocarcinoma | 12 | 27.2 |
| Signet ring cell adenocarcinoma | 2 | 4.5 |
Table 2: Histopathological types of CRC in the studied cases.
| Stages | No. of cases (44) | Percentages | |
|---|---|---|---|
| TNM | Tis | 7 | 15.9 |
| I | 4 | 9 | |
| II A | 14 | 61.6 | |
| IIB | 19 | 43.1 | |
| Duke's | A | 3 | 6.8 |
| B | 34 | 77.2 | |
| C | 0 | - | |
| tis | 7 | 15.9 | |
| MAColler's | A-B1 | 9 | 20.4 |
| B2 | 14 | 61.6 | |
| B3 | 21 | 47.7 | |
Table 3: Stages of CRC in the studied cases.
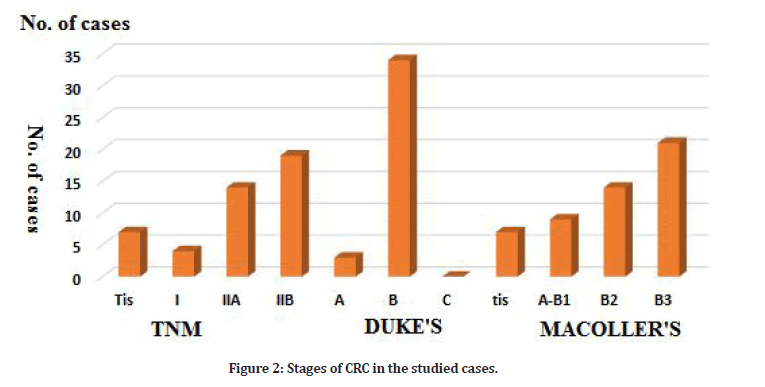
Figure 2: Stages of CRC in the studied cases.
| Variable | Cases No. | microRNA-92a | KWT test | P value | |||
|---|---|---|---|---|---|---|---|
| Mean | ± SD | Range | |||||
| Type of CRC | Adenocarcinoma | 30 | 7.07 | 0.21 | 6.46-7.29 | 5.17 | 0.075 |
| Mucinous adenocarcinoma | 12 | 6.86 | 0.26 | 6.47-7.28 | |||
| Signet ring cell adenocarcinoma | 2 | 6.89 | 0.13 | 6.99-7.21 | |||
Table 4: Expression of Serum microRNA – 92a (miR-92a) level in the studied groups.
Discussion
The need for sensitive, specific, affordable, noninvasive, and technically practical early detection and diagnosis approaches is urgent, given the high prevalence of CRC morbidity and mortality [13]. Most cases of CRC develop from polyps. There are three different kinds of polyps: adenomatous polyp, hyperplastic polyp, and junior polyp called hamartoma. Adenomatous is the only premalignant type; it may be tubular, villous, or tubulovillous, with villous adenomas the most likely to be cancerous [14]. Colonoscopy has been the gold standard for CRC diagnosis up to this point, but its invasiveness prevents it from being widely used in clinical settings [15]. The miR-92a family members play important roles in the onset and progression of CRC and may act as cancer biomarkers [16]. However, it is still unknown how miR- 92a-1 functions as a diagnostic marker in CRC. In the current work, a ROC curve was created to investigate the diagnostic utility of miR-92a-1. Studies have shown that the patterns of miRNA expression vary between various tumor types and between normal tissue and malignant tissues [17]. The majority of miRNA expression studies have been conducted on tissue samples. Tumor-derived miRNAs would be present in blood and appear to be stably protected from endogenous ribonuclease activity in circulation; hence the diagnostic and prognostic potential for circulating miRNAs was assumed [18].
Turchinovich, et al. [19] found that both adenomas and carcinomas have increased levels of miR-92a transcription. They demonstrated that miR-92a was essential for the growth of colorectal carcinoma as it specifically targeted the antiapoptotic molecule BCL-2- interacting mediator of cell death in colon cancer. For all these considerations, the aim of the present study was to assess serum miR-92a expression level as a stable bloodbased biomarker for the detection of CRC.
In the current study, serum miR-92a levels were significantly higher (P ˂ 0.001) in the studied cases with CRC than in the control group. This was in agreement with [20], who found that miR-92a levels were significantly elevated in serum of CRC patients compared to healthy controls. Additionally, according to Huang and colleagues, advanced adenoma and colorectal cancer patients can be distinguished from healthy controls by circulating miR92a [12]. Furthermore, miR92a had a sensitivity of >62% and a specificity of >84% for separating advanced adenoma and colorectal cancer from healthy individuals [21].
Conclusion
There is an urgent need for a gold standard for the early identification and non-invasive diagnosis of CRC. MiR-92a serum levels were noticeably higher in the cases group. Serum levels of miR-92a showed a significant positive correlation with both TNM and MAColler stages. Further extended studies on larger numbers of CRC patients, comparing micR-92a with other markers, are needed. Follow-up of patients is important to assess serum miR- 92a level in response to therapeutic maneuver.
Acknowledgements
This publication was supported by the Deanship of Scientific Research at Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia. In addition, we thank those who participated and contributed to the study.
Authors Contributions
All authors contributed to the research and/or preparation of the manuscript. Ali Hassan A. Ali, Mohammed H. Karrar Alsharif, and Mohammed Mobarak M. Almudarra participated in the study design and wrote the first draft of the manuscript. Turkey Saad AlGraene, Ahmed M Alsomali, and Moataz Daadour collected and processed the samples. Yousef M Alsomali and Mohammed Abdullah Aldossari participated in the study design and performed the statistical analyses. All of the authors read and approved the final manuscript.
Funding
This study has not received any external funding.
Conflicts of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of Data and Materials
The data are available upon request from the authors.
Ethics Approval
All series of steps that were implemented in this study that included animal models complied with the Ethics Committee of Prince Sattam bin Abdulaziz University Institutional Review Board (PSAU-2022 ANT 79/43PI).
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87-108.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 7:209-249.
- Murphy G, Devesa SS, Cross AJ, et al. Sex disparities in colorectal cancer incidence by anatomic subsite, race and age. Int J Cancer 2011; 128:1668-1675.
- Bullard Dunn KM, Rothenberger DA. Colon, rectum, and anus. Schwartz's principle of surgery, 9th Edn. by Charles brunicardi, McGraw- Hill 2010; 29:1055-1117.
- Mysliwiec PA, Cronin KA, Schatzkin A, et al. New malignancies among cancer survivors: SEER cancer registries. 2006; 1973â??2000.
- Herreros-Villanueva M, Duran-Sanchon S, MartÃn AC, et al. Plasma MicroRNA signature validation for early detection of colorectal cancer. Clin Transl Gastroenterol 2019; 10:e00003.
- Croce CM, Calin GA. miRNAs, cancer, and stem cell division. Cell 2005; 122:6-7.
- Aslam MI, Taylor K, Pringle JH, et al. MicroRNAs are novel biomarkers of colorectal cancer. Br J Surg 2009; 96:702-710.
- Caldarella A, Crocetti E, Messerini L, et al. Trends in colorectal incidence by anatomic subsite from 1985 to 2005: A population-based study. Int J Colorectal Dis 2013; 28:637-641.
- Sandhu S, Garzon R. Potential applications of microRNAs in cancer diagnosis, prognosis, and treatment. Semin Oncol 2011; 38:781-787.
- Wu CW, Ng SS, Dong YJ, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut 2012; 61:739-745.
- Huang Z, Huang D, Ni S, et al. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int J Cancer 2010; 127:118-126.
- Elaguizy M, Sheta M, Ibrahim N, et al. Serum microRNA-18a, MicroRNA-21 and microRNA-92a as diagnostic markers in colorectal cancer patients. J Buon 2020; 25:1443-1448.
- Shi Y, Liu Z. Serum miR-92a-1 is a novel diagnostic biomarker for colorectal cancer. J Cell Mol Med 2020; 24:8363-8367.
- Du M, Liu S, Gu D, et al. Clinical potential role of circulating microRNAs in early diagnosis of colorectal cancer patients. Carcinogenesis 2014; 35:2723-2730.
- Elshafei A, Shaker O, Abd El-Motaal O, et al. The expression profiling of serum miR-92a, miR-375, and miR-760 in colorectal cancer: An Egyptian study. Tumour Biol 2017; 39:1010428317705765.
- He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature 2007; 447:1130-1134.
- Wang LG, Gu J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol 2012; 36:61-67.
- Turchinovich A, Weiz L, Langheinz A, et al. Characterization of extracellular circulating microRNA. Nucleic Acids Res 2011; 39:7223-7233.
- Liu GH, Zhou ZG, Chen R, et al. Serum miR-21 and miR-92a as biomarkers in the diagnosis and prognosis of colorectal cancer. Tumour Biol 2013; 34:2175-2181.
- Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: A potential marker for colorectal cancer screening. Gut 2009; 58:1375-1381.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ali Hassan A Ali1,2*, Mohammed H Karrar Alsharif1, Mohammed Mobarak M Almudarra3, Turky Saad AlGraene3, Moataz Daadour3, Yousef M Alsomali3, Mohammed Abdullah Aldossari3 and Ahmed M Alsomali3
1Department of Anatomy, College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, KSA2Department of Anatomy, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
3College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj, KSA
Received: 23-Aug-2022, Manuscript No. jrmds-22-75681; , Pre QC No. jrmds-22-75681(PQ); Editor assigned: 02-Aug-2022, Pre QC No. jrmds-22-75681(PQ); Reviewed: 09-Sep-2022, QC No. jrmds-22-75681(Q); Revised: 13-Sep-2022, Manuscript No. jrmds-22-75681(R); Published: 20-Sep-2022
