Research - (2021) Volume 9, Issue 5
Comparative Clinicopathological and Immunohistochemical Study of Oral Schwannomas and Neurofibromas
Awf SH Mahmood1, Bashar H Abdullah1, Omar Museedi1, Ameer DH Hameedi2 and Senol Dane2*
*Correspondence: Senol Dane, Department of Pathology, College of Medicine, University of Baghdad, Bab-Almoadham, Baghdad, Iraq, Email:
Abstract
It is essential to differentiate between oral schwannomas and neurofibromas. Depending on the neoplasm type, the surgeon can decide the optimal treatment approach. Additionally, the potential of malignant transformation and the association with neurofibromatosis type1 should be considered in neurofibromas. Twenty-two cases diagnosed as schwannomas and neurofibromas were retrieved from the Pathology Laboratory at the College of the Dentistry/ University of Baghdad. Immuno-histochemical analysis was performed using antibodies S-100, EMA, and Calretinin. The mean age of the patients with neurofibroma was 38.83, whereas it was 29.5 among those with schwannoma. Women were more frequently affected by schwannoma, while no gender predominance was noted in neurofibroma cases. The tongue was the most prevalent site for schwannoma, while the alveolar ridge was the most common neurofibroma location. The immunohistochemical analysis showed a stronger and more diffuse immunoreactivity of the S-100 in schwannomas than neurofibromas. The EMA reactivity was absent in most neurofibromas, while a weak expression was observed in schwannomas, especially by the capsular area. Moreover, a variable reactivity to calretinin was demonstrated by schwannomas; however, an absence of expression in neurofibroma was noted. We concluded that combined immunohistochemical evaluation of S-100, EMA, and Calretinin could be regarded as a reliable method to differentiate between them.
Keywords
Neurofibroma, Schwannoma, S-100, EMA, Calretinin
Introduction
Schwannoma and neurofibroma tumors are the most common nerve sheath peripheral tumors [1,2], and although these tumors constitute the largest number of the neurogenic neoplasms, they rarely affect the oral cavity [3–6]. Neurofibroma consists of a mixture of cells, including Schwann cells, perineural cells, fibroblasts, and mast cells [2,4]. Alternatively, Schwannoma (neurilemmoma) is com-posed exclusively of Schwann cells [2,7,8].
Schwannoma is usually a solitary, asymptomatic, slowly growing lesion that equally affects both genders and has a mean age of twenty to fifty years. Some diseases, such as type 2 neurofibromatosis and schwannomatosis, may occur with multiple unilateral or bilateral vestibular nerve schwannomas as a feature [4,9]. In the oral cavity, the tongue is the most frequent site of involvement by schwannomas [10,11].
Histologically, schwannoma is usually encapsulated. The tumor cells are arranged into two different organization patterns: Antoni A, consisting of spindle-shaped cells with an elongated nucleus, is arranged in palisades or separated parallel rows through an acellular eosinophilic area called (Verocay body).
Furthermore, Antoni B, which is characterized by spindle-shaped cells scattered in a loose connective tissue matrix in a disorganized manner where microcystic degeneration and inflammatory cells can be detected [12].
Neurofibromas are the most common benign neoplasms that originate from the peripheral nerves [13] and occur as a single tumor or multiple ones [6] when associated with neurofibromatosis type 1 (NF-1) [14–19]. Neurofibromas mainly affect individuals of twenty to forty years or younger when related with NF-1 [6,14–19].
Solitary neurofibromas are usually painless and slow-growing of varying sizes. These tumors appear after puberty and remain progressive throughout life [6]. Deeper and visceral or plexiform neurofibroma can be observed as part of NF-1. The malignant transformation potential is more prevalent in neurofibromas than in schwannomas [6,17–24]. The tongue, buccal mucosa, and lips are oral sites that are more commonly affected by neurofibroma [6,25]. Due to the neurofibromas derived from Schwann cells, perineural cells, and fibroblasts, the neurofibromas' microscopic aspects are of great cellular heterogeneity [3,4,6]. They are well-circumscribed tumors characterized by the intermingling of spindle-shaped cells with a wavy nucleus within a myxoid background consisting of delicate col-lagen fibers and a variable number of mast cells [6].
Although it is generally not difficult to differentiate between schwannomas and neurofibromas by standard light microscopy, there can be considerable morphological overlap between them in a few cases. The differentiation between these tumors is fundamental for the surgeon to determine the intended surgery of choice [6,26].
Schwannomas can be removed surgically without sacrificing the nerve, primarily if it arises from the nerve lining. However, for most neurofibromas, the nerve is part of the mass, and surgery involves resection and nerve grafting to maintain function [26,27]. Besides, neurofibromas have a small but non-negligible possibility for malignant transformation. This is more commonly associated with Neurofibromatosis Type 1 (NF-1) than with schwannomas [2,28].
Several immunohistochemical markers have been studied to differentiate these two entities, and varying sensitivities and specificities have been shown [1,26,29]; however, the utilization of these biomarkers alone or in combination may be inadequate as these tumors occasionally exhibit cytomorphological and immunohistochemical overlap [1,26].
In this study, typical cases of schwannomas and neurofibromas are investigated by immunohistochemical staining of calretinin, EMA, S-100 protein. We evaluated and compared these immuno-histochemical markers' expressions to have a reliable and useful method to differentiate between schwannomas and neurofibromas.
Materials and Methods
Oral lesions diagnosed as schwannomas and neurofibromas submitted to the biopsy service to Oral Pathology Laboratory at the College of Dentistry, the University of Baghdad between 2000 and 2019 were retrieved and reviewed. Clinical data (age, gender, site, size of the lesion, duration, type of the biopsy), laboratory records, and surgical notes were tabulated.
Four micrometer thick sections were taken from the archived paraffin blocks of the chosen cases and processed routinely for morphological and immunohistochemical evaluation. These sections were stained with hematoxylin and eosin (H&E) for morphological examination and analyzed under light microscopy. To certify histopathological diagnosis, a streptavidinbiotin- peroxidase complex Abcam® (Cambridge, UK) immunohistochemistry analysis and evaluation were performed for all cases. The dilution and clonal-ity of the antibodies (S-100, EMA, and Calretinin) used in this study are listed in (Table 1).
Table 1. Antibodies employed in the study and stated according to the manufacturer’s datasheet.
| Antibody | Manufacturer's code | Clonality | Isotype | Immunogen | Host | Applied dilution | Manufacturer |
|---|---|---|---|---|---|---|---|
| Anti-S100 antibody | ab136629 | Monoclonal | IgG | Synthetic peptide corresponding to Human S100 (C terminal). | Rabbit | 1/100- 1/200. | Abcam® |
| Anti-MUC1 antibody | ab15481 | polyclonal | IgG | Synthetic peptide within Human MUC1 aa 1200 to the C-terminus. | Rabbit | 1/100 | Abcam® |
| Anti-Calretinin antibody | Ab702 | polyclonal | IgG | Full-length protein corresponding to Calretinin | Rabbit | 1/50-1/100 | Abcam® |
The microscopic pattern related to the organization of tumor cells, the background matrix, and the exist-ence of inflammatory infiltrate for each schwannoma and neurofibromas cases were analyzed and record-ed were analyzed by two pathologists independently. The immunohistochemical assessment was primarily utilized to confirm the diagnosis by the percentage of immunoreactivity presented by tumor cells under light microscopy.
Results
Twenty-two patients with neurofibromas and schwannomas were retrieved in the mentioned period. Twelve cases of neurofibromas and ten cases of schwannomas were included in this study. The neurofibroma sample consisted of six females and six males, and their ages ranged from 29 to 64 years, with a mean age of 38.8 years. Among the schwannoma patients, there were seven females and three males. The patients' ages ranged from 14 to 48 years, with a mean age of 29.5 years. Regarding the site, the most common site of involvement of neurofibroma in the presented study was the alveolar ridge (33%), followed by palate (25%) and buccal mucosa (16.7%), whereas the tongue (33%) was the most common site of involvement for schwannoma followed by palate (25%), lower lip (16.7%), gingiva (8.3%), buccal mucosa (8.3%), and centrally within bone involving the body of the mandible (8.3%) as illustrated in (Table 2).
Table 2. Clinical characteristics of the neurofibromas and schwannomas
| Calretinin (%) | EMA (%) | S-100 (%) | Histological | Case No. |
|---|---|---|---|---|
| - | - | 30 | Solitary (Conventional) | 1 |
| - | - | 30 | Solitary (Conventional) | 2 |
| - | - | 80 | Cellular (Diffuse) | 3 |
| - | - | 30 | Solitary (Conventional) | 4 |
| - | 30 | 40 | Solitary (Conventional) | 5 |
| - | 10 | 30 | Solitary (Conventional) | 6 |
| - | - | 80 | Cellular (Diffuse) | 7 |
| - | - | 30 | Solitary (Conventional) | 8 |
| - | - | 40 | Solitary (Conventional) | 9 |
| - | - | 30 | Solitary (Conventional) | 10 |
| - | 40 | 40 | Plexiform | 11 |
| - | - | 30 | Solitary (Conventional) | 12 |
The microscopical findings and immunohistochemical (IHC) profiles of the neurofibromas and schwannomas are listed in (Tables 3 and 4), respectively. Histopathologically, nine cases of neurofibromas (75%) were of solitary (conventional) type characterized by circumscribed but nonencapsulated spindle cells, consisting of uniform and randomly distributed spindle cells. Tumors cells have wavy and hyperchromatic nuclei in a loose collagenous background. Two cases (16.7%) were diffuse or cellular variant, composed mainly of small-sized round to spindleshaped cells, with a honeycomb appearance and tend to in-filtrate subcutaneous fatty tissues. Furthermore, one case of a plexiform variant (8.3%) was detected.
Table 3. Microscopical findings and IHC profile of the neurofibroma.
| Size | Site | Age | Gender | Diagnosis | Case no |
|---|---|---|---|---|---|
| 8*4 cm | palate | 35 | M | Neurofibroma | 1 |
| 1.5*1 cm | palate | 64 | M | 2 | |
| 4*2 cm | Alveolar ridge | 41 | F | 3 | |
| 5*3 cm | Alveolar ridge | 29 | M | 4 | |
| 1*0.5 cm | Alveolar ridge | 48 | F | 5 | |
| 2*2 cm | Palate (Mid) | 32 | F | 6 | |
| 1.5*1 cm | Buccal mucosa | 39 | M | 7 | |
| 3*2 cm | Tongue | 41 | F | 8 | |
| 0.5 cm | Tongue | 36 | M | 9 | |
| 1*1 cm | Tongue | 35 | F | 10 | |
| 3*2 cm | Buccal mucosa | 31 | F | 11 | |
| 3*2 cm | Alveolar ridge | 37 | M | 12 | |
| 2*1 cm | Gingiva | 29 | M | Schwannoma | 1 |
| 0.5 cm | Lower lip | 42 | F | 2 | |
| 1*1 cm | palate | 17 | M | 3 | |
| 0.5 cm | palate | 28 | M | 4 | |
| 3*2 cm | Tongue | 18 | F | 5 | |
| 3.5*2 cm | palate | 14 | F | 6 | |
| 1.5*1 cm | Tongue | 25 | F | 7 | |
| 0.5 cm | Lower lip | 43 | F | 8 | |
| 1*0.5 cm | Body of mandible | 31 | F | 9 | |
| 2*1 cm | Tongue | 48 | F | 10 |
Table 4. Microscopical findings and IHC profile of the schwannomas
| Calretinin (%) | EMA (%) | S-100(%) | Histological | Case No. |
|---|---|---|---|---|
| 10 | 20 | 90 | Conventional | 1 |
| 10 | 10 | 90 | Conventional | 2 |
| 20 | 30 | 80 | Conventional | 3 |
| 10 | 10 | 90 | Conventional | 4 |
| 30 | 10 | 80 | Conventional | 5 |
| 30 | 20 | 80 | Ancient | 6 |
| 70 | 10 | 90 | Conventional | 7 |
| 30 | 30 | 80 | Plexiform | 8 |
| 30 | 10 | 70 | Conventional | 9 |
| 40 | 10 | 90 | Conventional | 10 |
On the other hand, there were ten cases of schwannoma in this study. Eight cases (80%) revealed a conventional feature of fascicles of spindle-shaped cells, arranged into a cellular area (Antoni A) around the acellular eosinophilic area (Verocay bodies) and fewer cellular zones in a partially myxomatous back-ground stroma. The cellular zones of conventional schwannomas vary from case to case. There was peri-vascular hyalinization, which leads to the thickening of the blood vessel walls in most typical or conventional schwannomas cases. One case (10%) was an ancient variant with degenerative changes microscopically, particularly inflammation, hemorrhage, nuclear atypia, and extensive cystic changes. Additionally, one case of plexiform variant was found, comprising (10%) of the overall sample size. This kind is generally mistaken for a plexiform neurofibroma. The Antoni A zone and the Verocay bodies provide for the distinction of these two individuals.
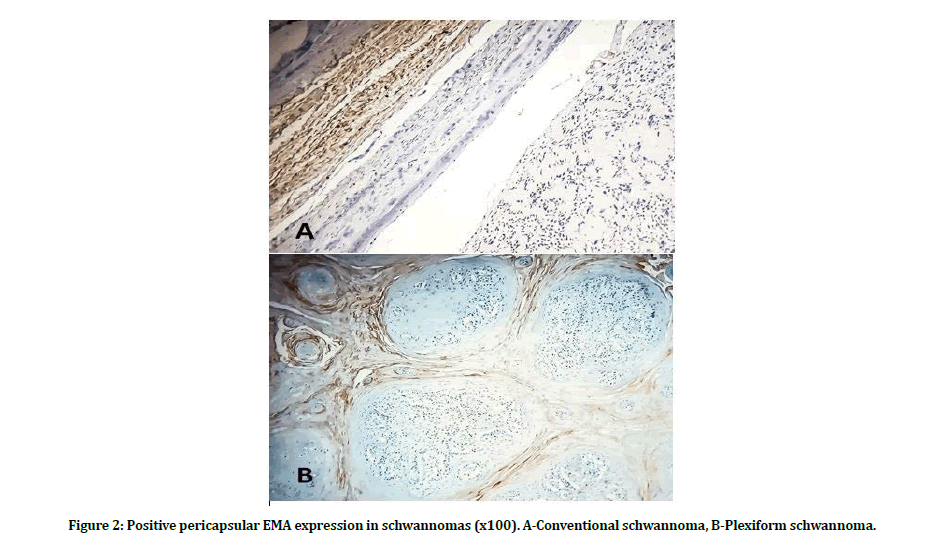
The immunohistochemical evaluation showed a stronger expression of S-100 marker for schwannoma cases as compared to neurofibromas. In all Schwannomas cases, we observed that strong and diffuse staining was detected in all schwannoma cells in all cases, while (75 percent) of cases revealed moderate staining and (25 percent) diffuse expression in neurofibroma as shown in (Figure 1). Moreover, all schwannoma cases exhibited a weak expression of EMA marker, particularly in the capsular area rather than lesional cells, as illustrated in (Figure 2). In contrast, the absence of expression was noticed by most neurofibroma cases (84 percent) and variable expression in the rest (16 percent).
Figure 1: Immunohistochemical analysis of S-100 in Schwannomas and Neurofibromas showing a strong immunoreactivity (x 100). A-Conventional schwannoma, B-Plexiform neurofibroma.
Figure 2: Positive pericapsular EMA expression in schwannomas (x100). A-Conventional schwannoma, B-Plexiform schwannoma.
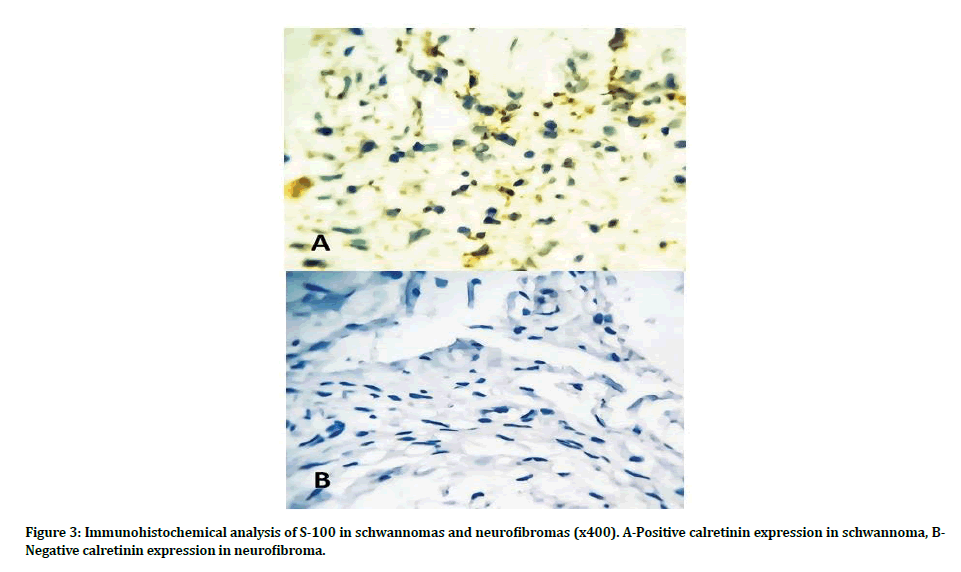
Furthermore, there was an absence of Calretinin expression demonstrated by neurofibroma cases. On the contrary, Calretinin expression for schwannomas revealed that forty percent of schwannomas cases had a weak expression, fifty percent of cases had a moderate expression, and only one case (ten percent) had a diffuse expression for this marker (Figure 3).
Figure 3: Immunohistochemical analysis of S-100 in schwannomas and neurofibromas (x400). A-Positive calretinin expression in schwannoma, BNegative calretinin expression in neurofibroma.
Discussion
This paper is a comparative study between the two most common peripheral nerve sheath tumors. The relation between the studied tumors and patients' gender is minimal as several works have shown that the gender difference is insignificant [6]. Regarding the studied sample, we observed that females were more affected than men by schwannomas, while no sex predilection was noted in neurofibroma cases. Most of the neurofibromas are diagnosed between the ages of twenty and forty years; however, these neoplasms occur at a younger age when associated with neurofibromatosis type 1 syndrome [5,30]. The mean age of seven documented literature reports of 127 cases of neurofibroma has been documented to be 37.8 years [6], which is remarkably close to the average age of 38.8 years reported in the study described. However, schwannomas mostly occur in patients between the age of twenty and fifty years. The peak incidence of schwannoma occurs in the third decade of life, and the mean age for this tumor is about 32 years [12], which is close to the mean age of 29.5 years documented in this report.
The most prevalent location for oral neurofibromas is the alveolar ridge, followed by the tongue and the palate, while the most frequent for schwannomas is the tongue, especially in the lateral and dorsal part of the tongue [5], which agrees with this presented study. Immunohistochemistry is a joint aid in the diagnostic study of spindle cell tumors. Reactivity for S-100 has been highlighted as the most relevant finding to corroborate neurogenic origin. S-100 revealed strong reactivity with schwannomas and variable evident positivity than neurofibromas, not more than 40% of cells in the presented study. The S-100 antigen has strong positivity to Schwann cell origin, which is the primary cell type schwannomas.
In contrast, neurofibromas contain cells with clear schwannian differentiation intermixed with perineurial and fibroblastic differentiation (Figure 1). Additionally, most neurofibromas lack EMA expression. On the contrary, schwannomas exhibit a weak positive immunoreactivity, especially in the pericapsular zones (Figure 2). Calretinin is a calcium-binding protein and belongs to the EF-hand protein family. The expression of calretinin is a hallmark of human mesothelioma and mesothelioma cells, certain types of epithelial and stromal ovarian cells [1]. In this study, all schwannomas demonstrate a positive reactivity to calretinin. On the contrary, all neurofibromas exhibit a negative expression.
Therefore, calretinin can be considered as a specific biomarker for schwannomas (Figure 3).
Conclusion
This study provides a strong clue that combined immunohistochemical staining with S-100, EMA, and calretinin, in addition to the microscopical evaluation, can be regarded as a reliable method to differentiate between schwannomas and neurofibromas. Besides, Calretinin can be considered as an excellent tool in distinguishing these tumors, especially in cases where a histological overlap is evident.
Ethical Approval
All experimental protocols were approved by the College of Dentistry, University of Baghdad. All experiments were carried out following the approved guidelines. (Ref no.167719 on 31/12/2019).
Financial Support
There was no financial disclosure.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Fine SW, Mcclain SA, Li M. Immunohistochemical Staining for Calretinin Is Useful for Differentiating Schwannomas From Neurofibromas. Am J Clin Pathol 2004;122:552–9.
- Guedes-Corrêa J, Cardoso R. Immunohistochemical Markers for Schwannomas, Neurofibromas and Malignant Peripheral Nerve Sheath Tumors—What Can the Recent Literature Tell Us? Arq Bras Neurocir Brazilian Neurosurg 2018;37:105–12.
- Chrysomali E, Papanicolaou SI, Dekker NP, Regezi JA. Benign neural tumors of the oral cavity: A comparative immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1997;84:381–90.
- Salla JT, Johann ACBR, Garcia BG, Aguiar MCF, Mesquita RA. Retrospective analysis of oral peripheral nerve sheath tumors in Brazilians. Braz Oral Res 2009;23:43–8.
- Oliveira FAF, Fernandes CP, Mota MRL, Sousa FB, De Freitas E Silva MR, Teófilo CR, et al. Expression of S-100, EMA, CD34 and presence of mast cells in eight oral neurofibromas, and a review of 127 cases of the literature. J Bras Patol e Med Lab 2013;49:347–54.
- do Nascimento GJF, de Pires Rocha DA, Galvão HC, de Lopes Costa AL, de Souza LB. A 38-year review of oral schwannomas and neurofibromas in a Brazilian population: Clinical, histopathological and immunohistochemical study. Clin Oral Investig 2011;15:329–35.
- Le LQ, Liu C, Shipman T, Chen Z, Suter U, Parada LF. Susceptible stages in Schwann cells for NF1-associated plexiform neurofibroma development. Cancer Res 2011;71:4686–95.
- Aswath N, Manigandan T, Sankari S, Yogesh L. A rare case of palatal schwannoma with literature review. J Oral Maxillofac Pathol 2019;23:36.
- Baser ME, Friedman JM, Evans DGR. Increasing the specificity of diagnostic criteria for schwannomatosis. Neurology 2006;66:730–2.
- Arda HN, Akdogan O, Arda N, Sarikaya Y. An unusual site for an intraoral schwannoma: A case report. Am J Otolaryngol - Head Neck Med Surg 2003;24:348–50.
- Thompson LDR, Koh SS, Lau SK. Tongue Schwannoma: A Clinicopathologic Study of 19 Cases. Head Neck Pathol 2019:1–6.
- Tamiolakis P, Chrysomali E, Sklavounou-Andrikopoulou A, Nikitakis NG. Oral neural tumors: Clinicopathologic analysis of 157 cases and review of the literature. J Clin Exp Dent 2019;11:e721–31.
- De Luca-Johnson J, Kalof AN. Peripheral nerve sheath tumors: an update and review of diagnostic challenges. Diagnostic Histopathol 2016;22:447–57.
- Marocchio LS, Oliveira DT, Pereira MC, Soares CT, Fleury RN. Sporadic and multiple neurofibromas in the head and neck region: A retrospective study of 33 years. Clin Oral Investig 2007;11:165–9.
- Jordan RCK, Regezi JA. Oral spindle cell neoplasms: A review of 307 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:717–24.
- Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, et al. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. J Am Med Assoc 1997;278:51–7.
- Yohay K. Neurofibromatosis types 1 and 2. Neurologist 2006;12:86–93.
- Gerber PA, Antal AS, Neumann NJ, Homey B, Matuschek C, Peiper M, et al. Neurofibromatosis. Eur J Med Res 2009;14:102–5.
- McClatchey AI. Neurofibromatosis. Annu Rev Pathol Mech Dis 2007;2:191–216.
- Furniss D, Swan MC, Morritt DG, Lim J, Khanna T, Way BLM, et al. A 10-year review of benign and malignant peripheral nerve sheath tumors in a single center: Clinical and radiographic features can help to differentiate benign from malignant lesions. Plast Reconstr Surg 2008;121:529–33.
- Odebode TO, Afolayan EAO, Adigun IA, Daramola OOM. Clinicopathological study of neurofibromatosis type 1: An experience in Nigeria. Int J Dermatol 2005;44:116–20.
- Ide F, Shimoyama T, Horie N, Kusama K. Comparative ultrastructural and immunohistochemical study of perineurioma and neurofibroma of the oral mucosa. Oral Oncol 2004;40:948–53.
- Apostolidis C, Anterriotis D, Rapidis AD, Angelopoulos AP. Solitary intraosseous neurofibroma of the inferior alveolar nerve: Report of a case. J Oral Maxillofac Surg 2001;59:232–5.
- Ferner RE, O'Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol 2002;15:679–84.
- Campos M, Fontes A, Marocchio LS, Nunes FD, De Sousa SCOM. Clinicopathologic and immunohistochemical features of oral neurofibroma. Acta Odontol Scand 2012;70:577–82.
- Park JY, Park H, Park NJ, Park JS, SungHJ, Lee SS. Use of calretinin, CD56, and CD34 for differential diagnosis of schwannoma and neurofibroma. Korean J Pathol 2011;45:30–5.
- Tsai WC, Chiou HJ, Chou YH, Wang HK, Chiou SY, Chang CY. Differentiation between schwannomas and neurofibromas in the extremities and superficial body: The role of high-resolution and color Doppler ultrasonography. J Ultrasound Med 2008;27:161–6.
- Yamaguchi U, Hasegawa T, Hirose T, Chuman H, Kawai A, Ito Y, et al. Low grade malignant peripheral nerve sheath tumour: varied cytological and histological patterns. J Clin Pathol 2003;56:826–30.
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin N Am 2004;15:157–66.
- Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med 2010;12:1–11.
Author Info
Awf SH Mahmood1, Bashar H Abdullah1, Omar Museedi1, Ameer DH Hameedi2 and Senol Dane2*
1Department of Oral Diagnosis, College of Dentistry, University of Baghdad, Bab-Almoadham, Baghdad, Iraq2Department of Pathology, College of Medicine, University of Baghdad, Bab-Almoadham, Baghdad, Iraq
Received: 05-Apr-2021 Accepted: 13-May-2021 Published: 20-May-2021