Research - (2022) Volume 10, Issue 11
Comparative efficacy and safety of the BioNTech Pfizer vaccine mRNA Covid-19 vaccine, a placebo-controlled trail in Mosul city
Ghaith Rabie Mohammed* and Ghada Younis Abdulrahman
*Correspondence: Ghaith Rabie Mohammed, College of Dentistry, University of Mosul, Iraq, Email:
Abstract
Background: Tens of millions of individuals have been affected by a global pandemic of the coronavirus diseases 2019 (Covid-19) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We desperately needed vaccinations that are both safe and effective, as soon as possible. Methods : We randomly assigned individuals 16 years of age and older to receive one dose, two doses, or three doses 21 days apart in one arm taking the BNT162b2 vaccine candidate (30 g per dose) to be followed prospectively, and a second arm of placebo to be followed retrospectively in an ongoing multinational, placebo-controlled, observer-blinded, pivotal efficacy trial. The SARS-CoV-2 full length spike protein is encoded by the nucleoside-modified RNA vaccine BNT162b2, which is packaged as a lipid nanoparticle. The vaccine's effectiveness against the incidence of laboratory-confirmed Covid-19 infections and safety were the main end objectives. Results: 75 people in all underwent randomization, and of them, 40 got injections of BNT162b2 and 35 were retrospectively followed to be a placebo. Participants assigned to receive one dose of BNT162b2 experienced 5 cases of Covid-19 with onset at least 60 days up to 19 days later; participants assigned to receive two doses of BNT162b2 experienced one case; participants assigned to receive three doses of BNT162b2 experienced none; participants assigned to receive placebo experienced 21 cases. The extent of vaccination determines the degree of protection; individuals with three vaccination doses experienced 100% protection against reinfection within three days. Taking into account that the placebo group, separated into two subgroups-those who were hospitalized at the time of sampling and those who received their first dose of vaccination-had a 100% and 60% risk, respectively, of contracting an illness the first time and again. If we take into account the relative risk of having a second infection (RR=5.13 in favor of the placebo group), this translates to a five-fold increase in protection against reinfection compared to non-vaccinated individuals. Short-term, mild-to-moderate discomfort at the injection site, weariness, and headache were all part of BNT162b2's safety profile. The frequency of severe adverse events was minimal and comparable across the placebo and vaccination groups. Conclusions : A three-dose regimen of BNT162b2 offered 80.5% average protection against Covid-19 in people 16 years of age or older. Similar to previous viral vaccinations, safety was shown over a median of three months.
Keywords
Vaccine, COVID-19, Safety, Efficacy, Cumulative incidence, Reinfection, Morbidity, Mortality
Introduction
Since the World Health Organization classified the coronavirus disease 2019 (Covid-19) a pandemic on March 11, 2020, it has afflicted tens of millions of people worldwide [1,2]. The most vulnerable groups to Covid-19 and its consequences include older individuals, those with certain comorbid diseases, and front-line professionals. According to the findings, younger individuals and other groups become more and more infected with Covid-19 and SARS-CoV-2, respectively [3]. To bring the epidemic under control, which has had catastrophic effects on health, the economy, and society, preventive vaccinations that are safe and effective are urgently required. Clinical trials of the vaccine candidate BNT162b2 [4], a lipid nanoparticle formulation [5], nucleoside-modified RNA (modRNA) [6], encoding the SARS-CoV-2 full-length spike, modified by two proline mutations to lock it in the perfusion conformation, a phase 1 safety and immunogenicity results have already been presented [7].
Two 30μg doses of BNT162b2 produced strong antigenspecific CD8+ and Th1-type CD4+ T-cell responses as well as significant SARS-CoV-2 neutralizing antibody titers in tests performed in the United States and Germany among healthy men and women [8]. Despite older people having a weaker neutralizing response than younger adults, 30 μg of BNT162b2 induced 50% neutralizing geometric mean titers in both older and younger individuals that were higher than the geometric mean titer seen in a human convalescent serum panel. Additionally, the short-term local (i.e., injection site) and systemic reactions were mostly reflected by the reactogenicity profile of BNT162b2. The development of the BNT162b2 vaccine candidate into phase 3 was encouraged by these results.
Here, we provide data conclusion on the safety, immunogenicity, and effectiveness of 30 μg of BNT162b2 in preventing Covid-19 in people aged 16 and older. A collection data on the immunogenicity of vaccines and the longevity of the immune response to vaccination are still being collected, thus such data are probably supportive in this respect.
Materials and Methods
Study population
75 subjects were enrolled to the study, assigned to each of the study groups as 35 subjects to be followed retrospectively for a period of 3 month for any positive cases of COVID-19 and symptomatology of severe COVID-19, and 40 subjects to be randomized to three subgroups and followed prospectively for either single (20 subjects), double (13 subjects) or triple (7 subjects) vaccination doses 21 days apart (Table 1, Figures 1 to Figure 3).
| Study group | Vaccination status | Cumulative positive cases |
|---|---|---|
| 1st Vaccinated group Tα (n=40) | Subjects enrolled while attending to the mRNA BNT126b2b vaccination. | 6 cases were confirmed from 3 month prospective follow up |
| 7 subjects were taking the third booster shot | 0 from third booster group | |
| 13 persons were taking the second booster dose | 1 from the second booster group | |
| 20 persons were taking the first vaccination dose | 5 from the first vaccination shot | |
| 2nd Placebo Tβ (n=35) | 20 persons were taking prior to the first vaccination dose, retrospectively investigated by questionnaire method. | 21 cases were confirmed from 3-month retrospective investigation |
| 15 COVID -19 positive cases not vaccinated before enrollment from quarantine hospital and retrospectively investigated by questionnaire method | 6 from vaccination 1st dose group | |
| 15 from the quarantine hospital group | ||
Table 1: Groups population review.
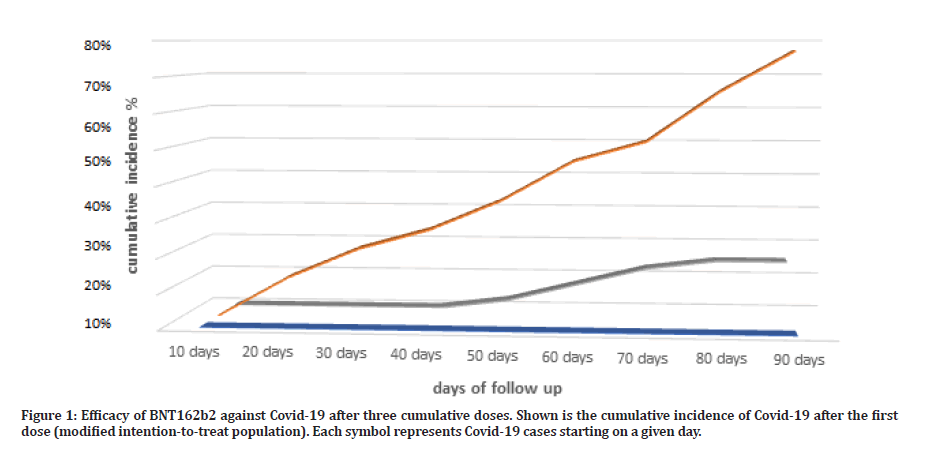
Figure 1: Efficacy of BNT162b2 against Covid-19 after three cumulative doses. Shown is the cumulative incidence of Covid-19 after the first dose (modified intention-to-treat population). Each symbol represents Covid-19 cases starting on a given day.
Inclusion and exclusion criteria
Subjects from 16 to 75 years old was selected, and based on past medical history all subjects with stable chronic disease were included and participants in the experiment could not have had an infection with the hepatitis B, hepatitis C, or human immunodeficiency virus (HIV). Prospective follow up study group, all subjects must have a negative COVID-19 history at least 60 days before enrolment to the trial, and must be free from serious medical condition at time of enrollment to eliminate study outcome bias. In retrospective follow up group, all subjects must be free from severe debilitating COVID-19 infection, not having a poorly controlled chronic medical condition, and fit to participate in the study, as well as a vaccination negative history prior to enrollment to the study. Both prospective and retrospective study groups members must not immunocompromised, taking steroids, or any immunosuppressant’s medication throughout the treatment period.
Study design
75 members were enrolled to two study groups, first the prospective study group, included 40 subjects subdivided into 20 subjects to take the first vaccination dose then phone numbers were taken and patients full information was taken to ensure meeting the inclusion exclusion criteria, follow up for 3 months following the single dose were performed to document the results and perform the proper analysis, other 13 subjects were assigned to the two vaccination dose this time 25 days apart, also followed prospectively to make sure of the results and interpret the outcomes, finally 7 members were enrolled to the three vaccination group, with 25 days between the doses, all outcomes are closely followed to make an accurate documentation of the results, and clarify the prospective data as a graph.
35 subjects represented the placebo comparative arm in this trail. 15 subjects were enrolled randomly from COVID-19 quarantine ward at al Shifaa hospital in Mosul, those were severely debilitated were not enlisted in this randomization. All recruited subjects had a negative vaccination status before enrolment. 20 subjects were enrolled from AlSalam teaching hospital, vaccination ward, prior to taking the first dose of vaccination. In both subgroups a deep questioning technique was performed to clarify the past vaccination, medical, genetic, infectious as well as COVID-19 status in the past 3-month period of the study, to make a clear interpretation of the collected data in hand, to reflect a clear retrospective history of the results.
Following up of cases and documentation in the prospective group was performed by routine communication via a phone number and messages, of any presented reactions, signs and symptoms, and document all possible cases of COVID-19 via genomic sequencing of the suspected cases and make the proper documentation. Follow up of all medication from antipyretics, analgesic to antibiotic use to muscle relaxants. Following up the retrospective study group, involves the questioning and close probing of the participants for the signs and symptoms of COVID-19 in the past 3 month prior for questioning, as well as positive documented cases of COVID-19 via NAAT or serology, the vaccination status, the medication, the chronic disease status of the participants.
Data set from both groups are gathered and uniformly placed in a statistical chart to facilitate data extraction and interpretation. All cases are strictly investigated to check wither they meet the inclusion or exclusion criteria prior to proceeding with statistical analysis and results.
Data analysis and statistics
Charting of the extracted data is performed via Microsoft Excel V-16.0, the number of cases per month were placed according to timeline per days of the intended study period of 3 months as 10 days gap of collection of outcomes. The percentage of case incidence from the total number of subjects in the given group was performed for each time period, and then the cumulative incidence over the entire period was taken by sum of the percentage incidence over all periods until the end of the proposed time of the study of 3 months. The comparative statistics between the two main study groups was performed via the Relative risk ratio analysis (RRR), between the cumulative percentage of incidence of COVID-19 cases in prospective vaccination groups in total and the corresponding retrospective study of placebo control. The vaccination efficacy was measure as the percentile gab of RRR between the two study groups. Cumulative percent of adverse effects was also measured for both groups, and RRR was then found to reflect significance of outcome, tracking the vaccine induced post injection adverse events and timing the of occurrence can add cumulative knowledge of the safety, tolerance and overall profile of adverse events associated with COVID-19 vaccination.
Results
Vaccination efficacy parameters
The prospective study groups, was subdivided into three groups. The single vaccination group, two vaccination group, and the three-vaccination group. The single vaccination group, subjects were taken from AlSalam vaccination ward upon first vaccination, were signed a patients information sheet and consent form detailing all study steps and procedures, this group were instructed to retake vaccination after 90 days to study the single dose efficacy as well as safety outcomes from vaccine administration.
The percentage COVID-19 case incidence in this group was 25% (5 out of 20 subjects), reflecting a roughly 75% protection from COVID-19 infection over a period of 90 days (Table 2). As compared to retrospective group the percentage COVID-19 case incidence was 77% (27 out of 35 subject) over the retrospective follow up period of 90 days, reflecting a roughly 23% protection from COVID-19 infection over the entire 90 days from protection free exposure time.
| Primary end point | BNT162b2 | Percentage incidence of cases | Placebo* | Percentage incidence of cases | RRR Vaccine Efficacy** | ||
|---|---|---|---|---|---|---|---|
| No. Of cases | Population | No. Of cases | Population | ||||
| Covid-19 occurrence 90 days after the first dose in participants without evidence of prior infection | 5 | 20 | 25% | 27 | 35 | 77% | 69.33% |
| Covid-19 occurrence 90 days after the second dose in participants without evidence of prior infection | 1 | 13 | 7.70% | 27 | 35 | 77% | 75% |
| Covid-19 occurrence 90 days after the third dose in participants without evidence of prior infection | 0 | 7 | 0% | 27 | 35 | 77% | 77% |
| * All cases of placebo were drawn from retrospective follow up group | |||||||
| ** RRR of protection % is the reverse of incidence %, and calculation is taken on this basis | |||||||
Table 2: Three-month follow-up for vaccine effectiveness against COVID-19.
The two-dose vaccination group, subjects were taken from AlSalam vaccination ward upon second vaccination dose, were signed a patients information sheet and consent form detailing all study steps and procedures, this group were instructed to either retake vaccination after 90 days or not, in order to study the two doses efficacy as well as safety outcomes from vaccine administration. The percentage COVID-19 case incidence in this group was 7.7% (1 out of 13 subjects), reflecting a roughly 92.3% protection from COVID-19 infection over a period of 90 days (Table 2). As compared to retrospective group the percentage COVID-19 case incidence was 77% (27 out of 35 subject) over the retrospective follow up period of 90 days, reflecting a roughly 23% protection from COVID-19 infection over the entire 90 days from protection free exposure time.
The triple vaccination group, subjects were taken from AlSalam vaccination ward upon third vaccination, were signed a patients information sheet and consent form detailing all study steps and procedures, this group were instructed to report vaccination side effects for 90 days follow up period, to have a complete triple dose efficacy as well as safety outcomes from vaccine administration. The percentage COVID-19 case incidence in this group was 0% (0 out of 7 subjects), reflecting a roughly 100% protection from COVID-19 infection over a period of 90 days (Table 2). As compared to retrospective group the percentage COVID-19 case incidence was 77% (27 out of 35 subject) over the retrospective follow up period of 90 days, reflecting a roughly 23% protection from COVID-19 infection over the entire 90 days from protection free exposure time.
Comparative parameter for vaccine efficacy is made utilizing the relative risk ratio, reflecting on the protection incidence from different number of vaccination doses compared to placebo. In the first dose vaccination group, the RRR was (69.33%) protection as compared to placebo group. This reflects a protective ratio for a single vaccination shot compared to placebo. If we consider the double vaccination regime, we find that the RRR was (75%) still reflects some superiority over single vaccination dose, and comparatively high superior protection compared to placebo arm. If we take the final three doses vaccination group, the RRR is (77%) this adds on to the single and double doses groups protection strength and yet give the superior protection as compared to the placebo arm.
Cumulative protection percentage as a mean from all vaccination doses protective percentages was (89.1%) this number reflects the overall vaccination group protection percent following vaccination as compared to placebo. If we consider the RRR for this percentage number as compared to placebo, it would be (74.2%) for all vaccination doses groups, this gives a collective superiority of vaccination over placebo under all terms and conditions. According to other analyses, vaccine effectiveness across subgroups characterized by age, sex, race, ethnicity, obesity, and the presence of a concomitant ailment was largely consistent with that seen in the general population (Tables 3 and 4).
| BNT162b2 | |||||||
|---|---|---|---|---|---|---|---|
| Study population | Number | 1st vaccine | % Case incidence | 2nd vaccine | % Case incidence | 3rd vaccine | % Case incidence |
| Total | 40 | ||||||
| Age | |||||||
| >15-50 | 22 | 2 | 5% | 0 | 0% | 0 | 0% |
| >50 | 8 | 1 | 2.50% | 0 | 0% | 0 | 0% |
| >60 | 6 | 2 | 5% | 1 | 2.50% | 0 | 0% |
| >75 | 4 | 0 | 0% | 0 | 0% | 0 | 0% |
| Gender | |||||||
| Male | 16 | 1 | 2.50% | 1 | 2.50% | 0 | 0% |
| Female | 24 | 4 | 10% | 0 | 0% | 0 | 0% |
| Illness | |||||||
| Hypertension | 5 | 1 | 2.50% | 0 | 0% | 0 | 0% |
| Diabetes | 9 | 3 | 7.50% | 1 | 2.50% | 0 | 0% |
| IHD* | 6 | 1 | 2.50% | 0 | 0% | 0 | 0% |
| RDs* | 3 | 0 | 0% | 0 | 0% | 0 | 0% |
Table 3: Population detailing three-month follow-up for vaccine effectiveness against COVID-19.
| Placebo | |||||||
|---|---|---|---|---|---|---|---|
| Study population | Number | 30 days | % Case incidence | 60 days | % Case incidence | 90 days | % Case incidence |
| Total | 35 | ||||||
| Age | |||||||
| >15-50 | 17 | 5 | 14.30% | 6 | 17.14% | 2 | 5.70% |
| >50 | 5 | 2 | 5.70% | 1 | 2.85% | 1 | 2.85% |
| >60 | 8 | 0 | 0% | 2 | 5.70% | 3 | 8.60% |
| >75 | 5 | 0 | 0% | 0 | 0% | 5 | 14.30% |
| Gender | |||||||
| Male | 16 | 2 | 5.70% | 5 | 14.30% | 4 | 11.42% |
| Female | 24 | 5 | 14.30% | 4 | 11.42% | 7 | 20% |
| Illness | |||||||
| Hypertension | 7 | 1 | 2.85% | 2 | 5.70% | 1 | 2.85% |
| Diabetes | 14 | 4 | 11.42% | 4 | 11.42% | 7 | 20% |
| IHD* | 11 | 2 | 5.70% | 3 | 8.60% | 2 | 5.70% |
| RDs* | 3 | 0 | 0% | 0 | 0% | 0 | 0% |
Table 4: Population detailing three-month follow-up for COVID-19 incidence in placebo group.
Vaccination safety parameters
Local reactions
There were 40 people in the subset of reactogenicity. Compared to placebo receivers, BNT162b2 recipients often reported higher local reactions. Less than 3% of participants across all age categories reported severe discomfort, with mild-to-moderate pain at the injection site within 7 days of an injection being the most often reported local reaction among BNT162b2 recipients (Figure 2A). Individuals over the age of 55 reported pain less frequently (64% after the first dosage and 47% after the second) than participants under the age of 55 (86% after the first dose and 79% after the second dose). Participants who reported redness or edema at the injection site were much less common. After the second dose, the percentage of participants reporting local reactions remained constant (Figure 2A), and no participant reported a grade 4 local reaction. Local reactions typically ranged from mild to moderate in severity and subsided within 1 to 2 days.
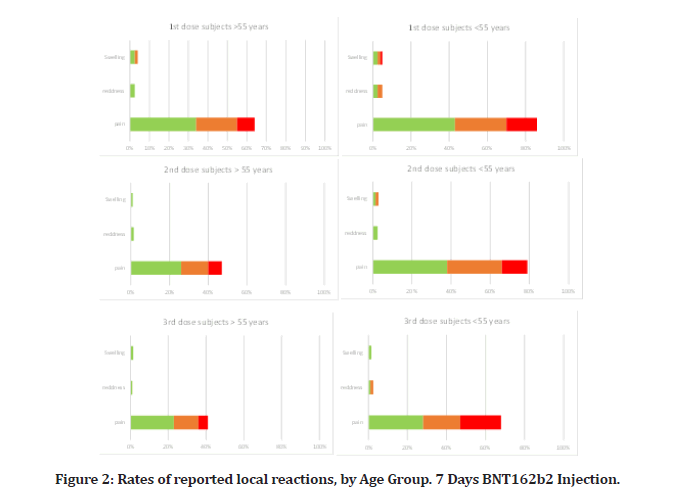
Figure 2: Rates of reported local reactions, by Age Group. 7 Days BNT162b2 Injection.
Systemic reactions
In the reactogenicity subgroup, younger vaccination receivers (16 to 55 years old) than older vaccine recipients (more than 55 years old) experienced systemic symptoms more often, and after dose 2, dose 3 than dose 1 (Figure 3). Although fatigue and headache were frequently reported by many placebo subjects (17% and 20%, respectively after the questionnaire, especially among younger subjects); and (14% and 11% among older subjects), they were the most frequently reported systemic events (47% and 61%, respectively, after the second dose, among younger vaccine recipients; 42% and 31%, among recipients who were older vaccine recipients). Slightly higher percent was reported for the third dose recipient (54% and 69%, respectively for younger subjects) and (51% and 37% for the older vaccine subjects). After the first dosage, there were a slight increase in fatigue and headache compared to what were reported in placebo subjects group following questionnaire, as (41% and 58% in younger subjects; and 39% and 27% in older subjects). There were no serious systemic events more often than 0.6%, except for a serious fatigue (4.1%) and serious headache (2.9%) after the second dose, and (4.7% and 3.2% respectively) after the third dose; severe systemic effects were only recorded in fewer than 1.8% of vaccination users.
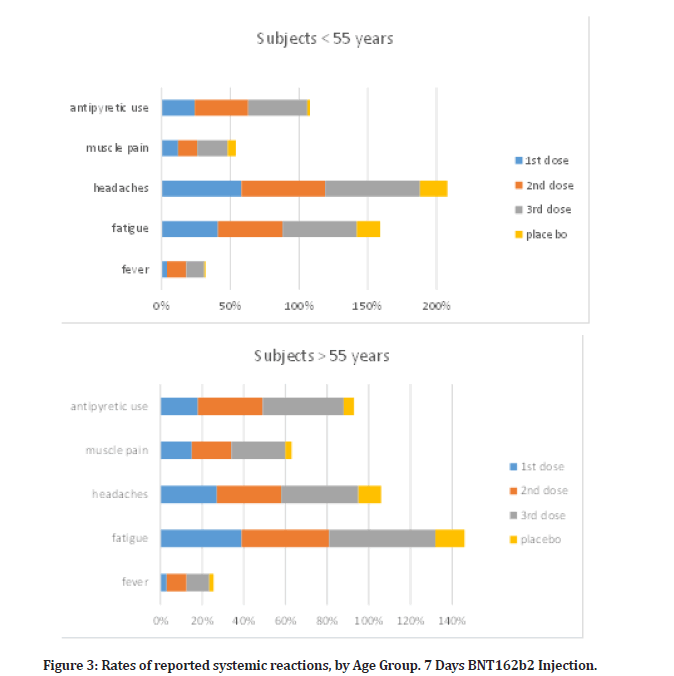
Figure 3: Rates of reported systemic reactions, by Age Group. 7 Days BNT162b2 Injection.
After the second dosage, 14% of younger and 9.5% of older vaccination recipients reported having a fever (temperature, 38°C). Third dose subjects had 13% and 11% respectively in such fever level. First dose subjects had a slight fever reaction in compare, as (4% in younger and 3% in older subjects), over all placebo subjects from questionnaire reported negligible fever as (2% in young and 1% in elderly).
Two members of the vaccination group noted a fever more than 40.0°C. Younger vaccine recipients had higher rates of antipyretic or pain medication use (24% after dose 1, 39% after dose 2, and 43% after dose 3) than older vaccine recipients (18% after dose 1, 31% after dose 2, and 39% after dose 3), while placebo subjects, regardless of age or dose, had lower rates of medication use (12–15%) than vaccine recipients, according to questionnaire. After immunization, systemic symptoms including fever and chills were sometimes seen for the first one to two days before going away.
Serious side effects
All 75 subjects who were recruited are given adverse event analyses, with varying levels of follow-up after dosage 1 (Table 2). BNT162b2 patients (23% vs. 16%, respectively) reported any adverse event or a connected adverse event (26% vs. 6%). Transient reactogenicity events, which were more often reported as adverse events by vaccination recipients than by placebo receivers, are mostly reflected in this distribution. (2.5%) of vaccination recipients with lymphadenopathy. Rarely did either group's members have severe adverse events, significant adverse events, or adverse events that required them to withdraw from the experiment. Among BNT162b2 recipients, there were two linked significant adverse events (shoulder injury related to vaccine administration, right axillary lymphadenopathy, paroxysmal ventricular arrhythmia, and right leg paresthesia). One of the placebo patients passed away (from respiratory distress)., while none of the BNT162b2 recipients did, the researchers did not believe that any fatalities were caused by the vaccination or a placebo. There was one fatality linked to Covid-19.
Discussion and Conclusion
The effectiveness of BNT162b2 against Covid-19 was determined to be 89.1% after three doses (30 μg each dosage), given 21 days apart. Both of the vaccine's key efficacy end goals were satisfied. These outcomes far beyond the minimal FDA authorization requirements while meeting our predetermined success criteria [9].
The point estimates of efficacy for subgroups based on age, sex, medication history, body mass index, or the presence of an underlying condition associated with a high risk of Covid-19 complications are also high, despite the fact that the study was not powered to conclusively assess efficacy by subgroup. More than 10 Covid-19 instances in any of the examined subgroups may provide insight into the subgroups' relationships to Covid-19 susceptibility. By 12 days after the first dose, or 7 days after the estimated median viral incubation period of 5 days, the cumulative incidence of Covid-19 cases over time among retrospective placebo and vaccine recipients starts to diverge [10], indicating the early onset of a partially protective effect of immunization. The effectiveness of a single-dose regimen was not intended to be evaluated in this research. Nevertheless, the observed vaccine efficacy against COVID-19 infection was 75% between the first and second doses, and it was 93.3% in the first 7 days following dose 2, reaching full efficacy against disease with onset at least 7 days after dose 2, and 100% in the first 7 days following dose 3. This indicates a sharp efficacy against COVID-19 infection. Only 5 of the 32 severe Covid-19 cases that were seen after the first dosage occurred in the group that received the vaccination. This result is in line with the strong comparative effectiveness overall versus all Covid-19 instances. The severe case split allays many of the theoretical worries about vaccine-mediated disease enhancement by providing early evidence of vaccine-mediated protection against serious illness [11]. BNT162b2's positive safety profile, which was discovered during phase 1 testing, 4,8 was verified throughout the phase 2 and phase 3 of the study. Similar to phase 1, reactogenicity was often low or moderate, and older persons had fewer and milder responses than younger adults. Local reactogenicity was equal after the three doses, while systemic reactogenicity was more frequent and severe after the second dosage than after the first dose. Nearly 44% of BNT162b2 users had severe fatigue, which is higher than that observed in recipients of some vaccinations suggested for senior citizens [12]. Additionally, this incidence of extreme fatigue is less frequent than that seen in older persons who received another viral vaccination that has been licensed for use [13]. Overall, reactogenicity occurrences were brief and ended a few days after they began. Lymphadenopathy, which typically went away after 10 days, was probably brought on by a strong immunological reaction to the vaccination. In the vaccination and retrospective placebo groups, the rate of major adverse events was comparable (0.5% and 0.35%, respectively).
There are a number of restrictions on this experiment and its early findings. The study has a greater than 45% probability of detecting at least one adverse event, if the true incidence is 0.01%, with an average of 40 participants per group in the subset of participants with a median follow-up time of three months after the first dose, but it is not large enough to reliably detect less common adverse events. This report covers the study participants' three-month follow-up after the first vaccination dosage. Therefore, it is yet unknown if side effects would manifest more than 3 to 4.5 months following the third dosage and how long protection will last. These findings do not address whether immunization prevents asymptomatic infection; a serologic end point (SARS-CoV-2 N-binding antibody) that may identify an infection history regardless of whether symptoms were present will be presented later [14]. Furthermore, at the time of this publication, it was not possible to establish a correlation of protection due to the high vaccination efficiency and the limited number of vaccine breakthrough cases. The prevention of Covid-19 in other groups, including as younger adolescents, children, and pregnant women, is not included in this paper.
When the vaccine is prepared for use, it may be kept for up to 5 days in a conventional refrigerator, but shipment and extended storage need very low temperatures [15]. The continuing stability investigations and formulation improvement, which may also be included in forthcoming reports, may reduce the need for the present cold storage requirement.
The relevance of the results in this research goes beyond the efficacy of the vaccine candidate. The results show that vaccination can prevent Covid-19 [16]. On January 10, 2020, the SARS-CoV-2 genomic sequence was made public by the Chinese Center for Disease Control and Prevention and distributed across the world under the GISAID (Global Project on Sharing All Influenza Data) initiative [17].
This marked the beginning of the creation of BNT162b2. Less than 11 months later, this thorough evaluation of safety and effectiveness shows how effective RNAbased vaccines may be in preventing pandemics and other infectious disease outbreaks [18]. These vaccines can be developed using just the viral genetic sequence information.
The BNT162b2 vaccination is authorized in the context of the present trial and will keep helping, together with other public health measures, to lessen the tragic loss of health, life, and economic and social well-being that has occurred as a consequence of the worldwide spread of Covid-19.
References
- https://coronavirus.jhu.edu/map.html.
- https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- Chen GL, Li XF, Dai XH, et al. Safety and immunogenicity of the SARS-CoV-2 ARCoV mRNA vaccine in Chinese adults: A randomized, double-blind, placebo-controlled, phase 1 trial. Lancet Microbe 2022; 3:e193-202.
- Kauffman KJ, Mir FF, Jhunjhunwala S, et al. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials 2016; 109:78-87.
- Karikó K. Modified uridines are the key to a successful message. Nat Rev Immunol 2021; 21:619.
- Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367:1260.
- Guerrera G, Picozza M, D’Orso S, et al. BNT162b2 vaccination induces durable SARS-CoV-2–specific T cells with a stem cell memory phenotype. Sci Immunol 2021; 6:eabl5344.
- https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained
- Cortés Martínez J, Pak D, Abelenda-Alonso G, et al. SARS-Cov-2 incubation period according to vaccination status during the fifth COVID-19 wave in a tertiary-care center in Spain: A cohort study. BMC Infect Dis 2022; 22:1-7.
- Li M, Wang H, Tian L, et al. COVID-19 vaccine development: Milestones, lessons and prospects. Signal Transduction Targeted Thera 2022; 7:1-32.
- Li AP, Cohen CA, Leung NH, et al. Immunogenicity of standard, high-dose, MF59-adjuvanted, and recombinant-HA seasonal influenza vaccination in older adults. NPJ Vaccines 2021; 6:1-2.
- https://www.fda.gov/vaccines-blood-biologics/vaccines/shingrix.
- Milani GP, Dioni L, Favero C, et al. Serological follow-up of SARS-CoV-2 asymptomatic subjects. Sci Rep 2020; 10:1-7.
- https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/storage.html.
- https://www.uptodate.com/contents/covid-19-vaccines
- https://www.cidrap.umn.edu/news-perspective/2020/01/china-releases-genetic-data-new-coronavirus-now-deadly
- Machado BA, Hodel KV, Fonseca LM, et al. The importance of RNA-based vaccines in the fight against COVID-19: An overview. Vaccines 2021; 9:1345.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ghaith Rabie Mohammed* and Ghada Younis Abdulrahman
College of Dentistry, University of Mosul, IraqReceived: 19-Oct-2022, Manuscript No. jrmds-22-80454; , Pre QC No. jrmds-22-80454(PQ); Editor assigned: 21-Oct-2022, Pre QC No. jrmds-22-80454(PQ); Reviewed: 04-Nov-2022, QC No. jrmds-22-80454(Q); Revised: 08-Nov-2022, Manuscript No. jrmds-22-80454(R); Published: 15-Nov-2022
