Research - (2022) Volume 10, Issue 1
Comparison of Haemoglobin, Red Cell Distribution Width and RBC Value in Normal and Oral Carcinoma Patients: A Retrospective Study
Harini M, Priyadharshini R* and Palati Sinduja
*Correspondence: Priyadharshini R, Department of Pathology, Saveetha Dental College and Hospitals, Saveetha Institutes of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, India, Email:
Abstract
Introduction: Oral squamous cell carcinoma is a cancer of the head and neck region affecting the various parts of the oral cavity such as the lining of the lips, mouth, upper part of the throat etc. Anaemia due to cancer comes under the category of anaemia caused due to chronic disease. Red Blood Cells are also known as erythrocytes, in most cancer patients are damaged due to the cancer treatments such as cancer chemotherapy, radiation therapy which damages and kills cancer cells. Studies regarding red cell count have concluded that there is a positive correlation between RBC count and cancer survival. Thus, this study aims at comparing and correlating the haemoglobin, RDW and RBC values of normal and oral squamous cell carcinoma patients. Materials and methods: Haematology reports of 10 normal patients and 10 oral squamous cell carcinoma patients were collected from Saveetha Dental College and Hospitals, Chennai from the patient record management system. The values of the parameters (haemoglobin, red cell distribution width and red cell value) were entered in an excel sheet and were imported to SPSS software for statistical analysis. The statistical test done was an independent t-test. Results and discussion: The data from the present study was collected and was statistically analysed using SPSS software. Inferring from the results of this study, the mean value of haemoglobin in the control group is 13.93 ± 2.8, the mean value of haemoglobin in the cancer group is 12.97 ± 5.0, the mean value of RDW in the control group is 12.51 ± 1.44, the mean value for RDW in cancer group is 12.86 ± 1.4, the mean value of RBC in the control group is 4.84 ± 0.9 and the mean value of RBC in cancer group is 4.59 ± 1.54. The P-value of haemoglobin is 0.135 (>0.05); p-value of RDW is 0.843 (0>0.05) and p-value of RBC is 0.429 (>0.05). Conclusion: This study showed no statistical significance among the haemoglobin, RDW and RBC values among cancer and control patients.
Keywords
Haemoglobin, RDW, RBC count, Novel method, Oral squamous cell carcinoma
Introduction
Oral squamous cell carcinoma is a cancer of the head and neck region affecting the various parts of the oral cavity such as the lining of the lips, mouth, upper part of the throat etc [1]. The carcinoma originates as a painless whitish patch which eventually develops to form a thickened red patch which continues to grow [2]. The main risk factors for oral cancer include tobacco, alcohol consumption, HPV infection, chewing betel nut and excess sun exposure. The treatments for oral cancer depend on the size of cancer, location and spread of cancer and it includes a combination of various surgeries such as radiation therapy, chemotherapy or targeted therapy [3]. Based on the epidemiology studies in 2012, more than three hundred patients have been affected with carcinoma in the oral cavity and the lip region which makes oral cancer one of the most prevalent cancers to occur [4].
Anaemia is the most related complication in all types of cancer and is known to reduce the quality of life [5]. Anaemia due to cancer comes under the category of anaemia caused due to chronic disease and this is a result of the production of disease-stimulated inflammatory cytokines such as interleukin-1, TNF (tumour necrosis factor), interferons etc. which leads to the inhibition of erythropoiesis due to the reduction in erythropoietin production and impairing the use of iron [6]. Increasing evidence has been obtained which suggests the correlation of anaemia and poor prognosis in cancer patients [7].
Red Cell Distribution Width (RDW) is a parameter that gives the size heterogeneity of the red blood cell and usually helps in differentiating the various types of anaemia [8]. RDW is also known to primarily reflect impaired erythropoiesis and abnormal red cell survival. It also correlates with other factors such as inflammation, nutritional disorders, impaired kidney function and prediction of various forms of carcinoma [9]. Studies conducted regarding the association of RDW with cancer resulted that there exists a positive correlation between a higher RDW value and increased mortality risk (for every 1% increase in RDW, the mortality rate increased by 14%) [10]. Red Blood Cells are also known as erythrocytes, in most cancer patients are damaged due to the cancer treatments such as cancer chemotherapy, radiation therapy which damages and kills cancer cells. Low levels of RBC often show symptoms like fatigue, shortness of breath, light-headedness, increased heart rate, headaches etc. Studies regarding red cell count have concluded that there is a positive correlation between RBC count and cancer survival [11]. Our team has extensive knowledge and research experience that has translated into high quality publications [12-31]. Thus, this study aims at comparing and correlating the haemoglobin, RDW and RBC values of normal and oral squamous cell carcinoma patients.
Materials and Methods
Haematology reports of 10 normal patients and 10 oral squamous cell carcinoma patients were collected from Saveetha Dental College and Hospitals, Chennai from the patient record management system. Before the initiation of the study, clearance was obtained by the Scientific Review Board with ethical approval number IHEC/SDC/BDS/1996/01. The values of the parameters (haemoglobin, red cell distribution width and red cell value) along with the age, gender, treatment is given, grading and staging of the cancer patients were entered in an excel sheet and were imported to SPSS software for statistical analysis. The statistical test done was an independent t-test.
Discussion
The data from the present study was collected and was statistically analysed using SPSS software. Inferring from the results of this study, the mean value of haemoglobin in the control group is 13.93 ± 2.8 and the mean value of haemoglobin in the cancer group is 12.97 ± 5.0 (Table 2) The mean difference is -0.960. Since there exists a mean difference between the control and the cancer groups, an independent t-test was done which revealed the p-value to be 0.135 (>0.05) (Table 3). Therefore, it is statistically insignificant. A study done by M Lind et al., comparing the haemoglobin level in cancer and control patients revealed that there is a marked reduction in the haemoglobin level of the cancer group compared to the control group (mean=10.66 ± 2.08) and the P-value <0.05, proving it statistically significant [32]. Groopman et al. conducted a similar study but concluded with contrasting results. The mean value of haemoglobin in cancer patients was found to be 12.56 ± 8.6 and the pvalue was greater than 0.05 deeming it statistically insignificant [33].
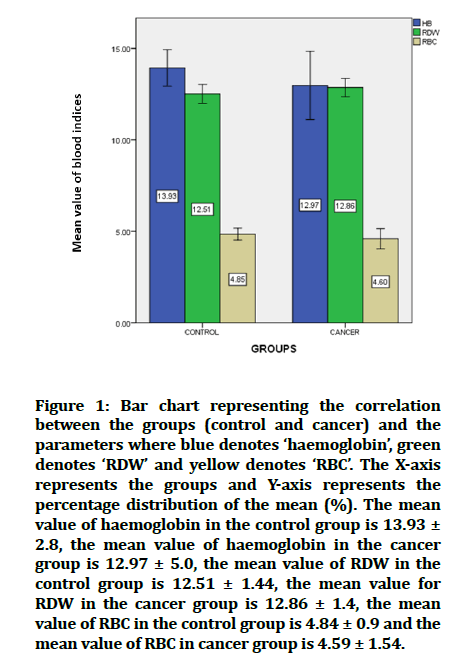
Figure 1: Bar chart representing the correlation between the groups (control and cancer) and the parameters where blue denotes ‘haemoglobin’, green denotes ‘RDW’ and yellow denotes ‘RBC’. The X-axis represents the groups and Y-axis represents the percentage distribution of the mean (%). The mean value of haemoglobin in the control group is 13.93 ± 2.8, the mean value of haemoglobin in the cancer group is 12.97 ± 5.0, the mean value of RDW in the control group is 12.51 ± 1.44, the mean value for RDW in the cancer group is 12.86 ± 1.4, the mean value of RBC in the control group is 4.84 ± 0.9 and the mean value of RBC in cancer group is 4.59 ± 1.54.
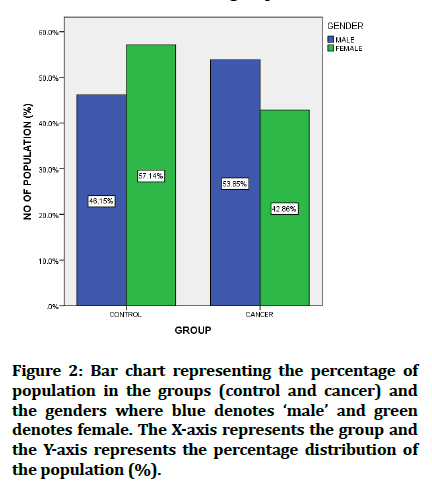
Figure 2: Bar chart representing the percentage of population in the groups (control and cancer) and the genders where blue denotes ‘male’ and green denotes female. The X-axis represents the group and the Y-axis represents the percentage distribution of the population (%).
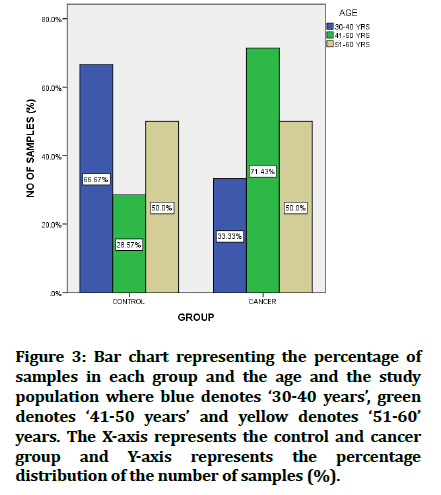
Figure 3: Bar chart representing the percentage of samples in each group and the age and the study population where blue denotes ‘30-40 years’, green denotes ‘41-50 years’ and yellow denotes ‘51-60’ years. The X-axis represents the control and cancer group and Y-axis represents the percentage distribution of the number of samples (%).
| Literature | Haemoglobin | RDW | RBC | P-Value |
|---|---|---|---|---|
| Qin, al. [34] | - | 13.78 ± 2.38 | - | P<0.05 |
| Lind et al. [32] | 10.66 ± 2.08 | - | - | P<0.05 |
| Brundha et al. [36] | - | - | 4.35 ± 1.1 | P>0.05 |
| Groopman et al. [33] | 12.56 ± 8.6 | - | 4.66 ± 15.2 | P>0.05 |
| Montagnana et al. [35] | - | 13.89 ± 1.34 | - | P<0.05 |
| Present Study | 12.97 ± 5.0 | 12.86 ± 1.4 | 4.59 ± 1.54 | P>0.05 |
Table 1: Table showing the haemoglobin, RDW, RBC and P values obtained from previous literature.
| Group statistics | |||||
|---|---|---|---|---|---|
| Groups | N | Mean | Std. Deviation | Std. Error mean | |
| HB | Control | 10 | 13.93 | 1.40004 | 0.44273 |
| Cancer | 10 | 12.97 | 2.59746 | 0.82139 | |
| RDW | Control | 10 | 12.51 | 0.72946 | 0.23068 |
| Cancer | 10 | 12.86 | 0.70427 | 0.22271 | |
| RBC | Control | 10 | 4.849 | 0.45598 | 0.14419 |
| Cancer | 10 | 4.595 | 0.77855 | 0.2462 | |
Table 2: showing the group statistics for haemoglobin, RDW and RBC value. The mean value of haemoglobin in the control group is 13.93 ± 2.8, the mean value of haemoglobin in the cancer group is 12.97 ± 5.0, the mean value of RDW in the control group is 12.51 ± 1.44, the mean value for RDW in the cancer group is 12.86 ± 1.4, the mean value of RBC in the control group is 4.84 ± 0.9 and the mean value of RBC in cancer group is 4.59 ± 1.54.
| Parameter | Independent T Test (P Value) |
|---|---|
| Haemoglobin | 0.135 |
| RDW | 0.843 |
| RBC | 0.429 |
Table 3: Shows the P-value of the parameter’s haemoglobin, RDW and RBC value. The P-value of haemoglobin is 0.135, the p-value of RDW is 0.843 and the p-value of RBC is 0.429.
The mean value of RDW in the control group is 12.51 ± 1.44and the mean value for RDW in the cancer group is 12.86 ± 1.4. The mean difference is 0.350. Since there exists a mean difference between the control and the cancer groups, an independent t-test was done which revealed the p-value to be 0.843 (>0.05) (Table 3). Hence, it is statistically insignificant. In a study on the value of red cell distribution width in patients with ovarian cancer and compared the RDW value of the cancer group and control group which resulted in the mean value of 13.78 ± 2.83 and a p-value of < 0.05 hence, statistically significant. [34]. (Table 1) A study by Martina Montagnana et al. done regarding the RDW in cancer and control patients resulted in a mean value of 13.89 ± 1.34 and p-value < 0.05. [35]. Hence, the RDW value among cancer and control group in both previous studies were statistically significant which is contrasting from the results obtained in this present study.
The mean value of RBC in the control group is 4.84 ± 0.9 and the mean value of RBC in the cancer group is 4.59 ± 1.54 (Table 2). The mean difference is -0.250. There exists a difference, hence an independent t-test was done which revealed the p-value to be 0.429 (>0.05). Hence, it is statistically insignificant. A study by M.P. Brundha et al. about the red cell count in cancer patients, resulted in a mean value of 4.35 ± 1.1 and p-value >0.05. Hence, it is statistically insignificant [36]. (Table 1) The mean value of RBC count in a study by Groopman et al. was found to be 4.66 ± 15.2 and a p-value >0.05 therefore it is statistically insignificant [33]. Hence, both the previous literature had concluded that the RBC value in cancer and control group was statistically insignificant.
This present study compared the values of the parameters, haemoglobin, RDW and RBC of cancer and control groups whereas the previous literature has discussed only any one of the parameters. This study had a smaller sample size of only 20 patients among which only 10 were oral cancer patients which is a limitation. Further, the surgical and chemotherapeutic details were not included in this study. In the future, further studies regarding these parameters and their significance in cancer patients could be done with a bigger sample size which could provide better and more accurate results.
Conclusion
In this study, the haemoglobin and RBC count of the cancer patients were decreased compared to the control group but were statistically insignificant. The RDW value of cancer patients was increased compared to the control group but also showed no statistical insignificance. In conclusion, this study showed no statistically significant difference among the haemoglobin, RDW and RBC values between cancer and control patients.
Acknowledgement
We thank Saveetha Institute of Medical and Technical Sciences, Saveetha Dental College and Hospitals, Saveetha University for supporting us to conduct the study.
Source of Funding
The present project is supported by
• Saveetha Institute of Medical and Technical Sciences, Saveetha Dental College and Hospitals, Saveetha University, India
• Sarkav Health Services, Chennai, India
References
- Sciubba JJ. Oral cancer. Am J Clin Dermatol 2001; 2:239–51.
- Marx RE, Stern D. Oral and maxillofacial pathology: A rationale for diagnosis and treatment. Quintessence Publishing 2012; 980.
- Goldenberg D, Lee J, Koch WM, et al. Habitual risk factors for head and neck cancer. Otolaryngology Head Neck Surg 2004; 131:986–993.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in Globocan 2012. Int J Cancer 2015; 136:E359–86.
- Mercadante S, Gebbia V, Marrazzo A, et al. Anaemia in cancer: Pathophysiology and treatment. Cancer Treatment Rev 2000; 26:303–11.
- Littlewood TJ. Erythropoietin for the treatment of anemia associated with hematological malignancy. Hematol Oncol 2001; 19:19–30. .
- Caro JJ, Jaime Caro J, Salas M, et al. Anemia as an independent prognostic factor for survival in patients with cancer. Cancer 2001; 91:2214–21.
- Salvagno GL, Sanchis-Gomar F, Picanza A, et al. Red blood cell distribution width: A simple parameter with multiple clinical applications. Critical Rev Clin Laboratory Sci 2015; 52:86–105.
- Ge W, Xie J, Chang L. Elevated red blood cell distribution width predicts poor prognosis in patients with oral squamous cell carcinoma. Cancer Management Res 2018; 10:3611–8.
- Patel KV, Ferrucci L, Ershler WB, et al. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Internal Med 2009; 169:515.
- Kos M, Hocazade C, Kos FT, et al. Evaluation of the effects of red blood cell distribution width on survival in lung cancer patients. Contemp Oncol 2016; 20:153–7.
- Anita R, Paramasivam A, Priyadharsini JV, et al. The m6A readers YTHDF1 and YTHDF3 aberrations associated with metastasis and predict poor prognosis in breast cancer patients. Am J Cancer Res 2020; 10:2546–54.
- Jayaseelan VP, Paramasivam A. Emerging role of NET inhibitors in cardiovascular diseases. Hypertens Res 2020; 43:1459–61.
- Sivakumar S, Smiline Girija AS, Vijayashree Priyadharsini J. Evaluation of the inhibitory effect of caffeic acid and gallic acid on tetR and tetM efflux pumps mediating tetracycline resistance in Streptococcus sp., using computational approach. J King Saud University Sci 2020; 32:904–9.
- Smiline Girija AS. Delineating the immuno-dominant antigenic vaccine peptides against gacs-sensor kinase in Acinetobacter baumannii: An in silico Investigational Approach. Front Microbiol 2020; 11:2078.
- Iswarya Jaisankar A, Smiline Girija AS, Gunasekaran S, et al. Molecular characterisation of csgA gene among ESBL strains of A. baumannii and targeting with essential oil compounds from Azadirachta indica. J King Saud University Sci 2020; 32:3380–7.
- Girija ASS. Fox3+ CD25+ CD4+ T-regulatory cells may transform the nCoV’s final destiny to CNS! J Med Virol 2020.
- Jayaseelan VP, Ramesh A, Arumugam P. Breast cancer and DDT: Putative interactions, associated gene alterations, and molecular pathways. Environ Sci Pollut Res Int 2021; 28:27162–73.
- Arumugam P, George R, Jayaseelan VP. Aberrations of m6A regulators are associated with tumorigenesis and metastasis in head and neck squamous cell carcinoma. Arch Oral Biol 2021; 122:105030.
- Kumar SP, Girija ASS, Priyadharsini JV. Targeting NM23-H1-mediated inhibition of tumour metastasis in viral hepatitis with bioactive compounds from Ganoderma lucidum: A computational study. Pharm Sci 2020; 82.
- Girija SA, Priyadharsini JV, Paramasivam A. Prevalence of carbapenem-hydrolyzing OXA-type β-lactamases among Acinetobacter baumannii in patients with severe urinary tract infection. Acta Microbiol Immunol Hung 2019; 67:49–55.
- Priyadharsini JV, Paramasivam A. RNA editors: Key regulators of viral response in cancer patients. Epigenomics 2021; 13:165–7.
- Mathivadani V, Smiline AS, Priyadharsini JV. Targeting Epstein-Barr virus nuclear antigen 1 (EBNA-1) with Murraya koengii bio-compounds: An in-silico approach. Acta Virol 2020; 64:93–9.
- Girija AS, Priyadharsini JV. Prevalence of Acb and non-Acb complex in elderly population with urinary tract infection (UTI). Acta Clin Belg 2021; 76:106–12.
- Anchana SR, Girija SAS, Gunasekaran S, et al. Detection of csgA gene in carbapenem-resistant Acinetobacter baumannii strains and targeting with Ocimum sanctum biocompounds. Iran J Basic Med Sci 2021; 24:690–8.
- Girija ASS, Shoba G, Priyadharsini JV. Accessing the T-cell and B-cell Immuno-dominant peptides from A.baumannii biofilm associated protein (bap) as Vaccine candidates: A computational approach. Int J Pept Res Ther 2021; 27:37–45.
- Arvind P TR, Jain RK. Skeletally anchored forsus fatigue resistant device for correction of Class II malocclusions-A systematic review and meta-analysis. Orthod Craniofac Res 2021; 24:52–61.
- Venugopal A, Vaid N, Bowman SJ. Outstanding, yet redundant? After all, you may be another Choluteca Bridge! Semin Orthod 2021; 27:53–6.
- Ramadurai N, Gurunathan D, Samuel AV, et al. Effectiveness of 2% Articaine as an anesthetic agent in children: Randomized controlled trial. Clin Oral Investig 2019; 23:3543–50.
- Varghese SS, Ramesh A, Veeraiyan DN. Blended module-based teaching in biostatistics and research methodology: A retrospective study with postgraduate dental students. J Dent Educ 2019; 83:445–50.
- Mathew MG, Samuel SR, Soni AJ, et al. Evaluation of adhesion of Streptococcus mutans, plaque accumulation on zirconia and stainless steel crowns, and surrounding gingival inflammation in primary molars: Randomized controlled trial. Clin Oral Investigations 2020; 24:3275–80.
- Lind M, Vernon C, Cruickshank D, et al. The level of haemoglobin in anaemic cancer patients correlates positively with quality of life. Br J Cancer 2002; 1243–12499.
- Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: Incidence and treatment. J National Cancer Institute 1999; 91:1616–34.
- Qin Y, Wang P, Huang Z, et al. The value of red cell distribution width in patients with ovarian cancer. Medicine 2017; 96:e6752.
- Montagnana M, Danese E. Red cell distribution width and cancer. Annals Translational Med 2016; 4:10–10.
- Brundha MP, Pathmashri VP, Sundari S. Quantitative changes of red blood cells in cancer patients under palliative radiotherapy-A retrospective study. Res J Pharm Technol 2019; 12:687.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Harini M, Priyadharshini R* and Palati Sinduja
Department of Pathology, Saveetha Dental College and Hospitals, Saveetha Institutes of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, IndiaReceived: 13-Dec-2021, Manuscript No. Jrmds-21-41705; , Pre QC No. Jrmds-21-41705 (PQ); Editor assigned: 15-Dec-2021, Pre QC No. Jrmds-21-41705 (PQ); Reviewed: 29-Dec-2021, QC No. Jrmds-21-41705; Revised: 03-Jan-2022, Manuscript No. Jrmds-21-41705 (R); Published: 10-Jan-2022
