Research - (2021) Volume 9, Issue 7
Compatibility of Novel Gingival Retraction Pastes Based on Boswellia papyrifera with Polyvinyl Siloxane Impression Material (A Comparative in-vitro Study)
Hiba A Salman1*, Manhal A Majeed2 and Eaman A Al-Rubaee1
*Correspondence: Hiba A Salman, Department of restorative and aesthetic dentistry, College of Dentistry, Baghdad University, Iraq, Email:
Abstract
This in-vitro study aimed to evaluate the compatibility of both conventional gingival retraction pastes based on Aluminium chloride (AlCl3), and novel gingival retraction pastes based on herbal astringent (Boswellia papyrifera (Bp) with and without AlCl3) with polyvinyl siloxane impression material by measuring the setting time polymerization index. The test of the Compatibility with Light body type III polyvinylsiloxane impression material (Variotime light body type III, KULZER, Germany) was carried out by measuring the standard-setting time of polyvinylsiloxane impression material to obtain ultimate compatibility time polymerization index (100%) for the control group and compared it with the compatibility time polymerization index of the impression after mixing with the studied gingival retraction pastes at oral temperature (35 ±0.1) ℃, rotational speed 0.5 rpm, and spindle size (0.7) using Brookfield HBDVII+ viscometer (USA). The total samples were thirty-two, eight for each group: Control group: polyvinylsiloxane impression without mixing with gingival retraction paste), Group 1: Novel gingival retraction paste (5% Bp+7.5% AlCl3) mixed with polyvinylsiloxane impression material, Group 2: Novel gingival retraction paste (5% Bp) mixed polyvinylsiloxane impression material, Group 3: Conventional gingival retraction paste (Astringent Retraction Paste, 3M ESPE, Germany) mixed with polyvinylsiloxane impression material. Statistical analysis was performed using non-parametric tests by SPSS software (IBM SPSS statistics version 25, IBM Corp., Chicago, USA), including the Kruskal Wallis H test (KWH) and Mann-Whitney U multiple comparisons test (MWU). This test revealed that the highest compatibility time polymerization index was seen in group 2. In contrast, the lowest compatibility time polymerization index was seen group 3 with significant differences, and there were no significant differences between group 1 and group 2. This study concluded that both novel gingival retraction pastes were higher Compatibility with polyvinylsiloxane impression material than the conventional gingival retraction pastes based on 15% AlCl3.
Keywords
Boswellia papyrifera, Astringent retraction paste, Polyvinylsiloxane impression, Novel gingival retraction paste, Compatibility with impression
Introduction
Gingival retraction techniques are widely used in fixed prosthodontics; their strategies are to reveal abutment margins before taking an impression for good marginal adaptation of the prosthesis and secure the biological space by reducing the chance of iatrogenic gingival recession [1]. Mechanical retraction, chemo mechanical retraction, displacement pastes, and surgical retraction techniques are some of the gingival displacement techniques available [2].
The positioning of the gingival retraction cord requires expertise and time, and anaesthesia is needed before it can be used. Positioning a gingival retraction cord around a tooth typically takes 7-10 minutes. According to records, if a gingival retraction cord was left in the gingival sulcus for longer than 15 minutes, it caused a 0.1mm marginal recession [3].
When a gingival retraction is required for many teeth at once, the problem becomes severe. As a result, cordless gingival retraction techniques have been revealed. The composition of gingival retraction paste should contain at least one clay, at least one astringent agent, and water, according to US patent No. 2008/0220050 A1.
An astringent agent is a chemical that causes body tissues to shrink or constrict. After topical application, this effect is normally local. Astringents are commonly used in gingival retraction procedures to avoid bleeding or oozing [4].
Aluminium chloride, Aluminium potassium sulphate, zinc chloride, ferric sulphate, epinephrine, and sympathomimetic amines are examples of astringent products of varying degrees of efficacy and protection.
However, these preferred chemo mechanical agents have their limitations, wherein Aluminium chloride has less vasoconstriction than epinephrine with the risk of sulcus contamination. It also modifies the surface detail reproduction and, most importantly, inhibits the set of polyvinylsiloxane and polyether impression [5].
Many astringent agents had pH values that were extremely low or acidic. The majority of the solutions were in the pH range of 1 to 3, which is higher than the etchants used in bonded composite resin restorations.
These solutions can harm oral tissues and have a greater impact; it would seem prudent to use caution when using low pH astringent agents and to avoid exposing sensitive intraoral tissues, particularly when tooth preparation is close to the dental pulp [6].
As a result, it is critical to reveal a new astringent agent for reducing the acidic activity in an aqueous base and fabricating a new gingival retraction paste with little or no Aluminum chloride that is easy to use, has good controllability, is affordable, and has higher biocompatibility and protection standards.
Plant products are thus appealing alternatives to synthetic products because of their biocompatibility, low toxicity, environmental friendliness, and low cost.
Herbs are widely being used as medicine, either as home remedies or as complementary and alternative medicines.
In this analysis, Boswellia papyrifera was used as a potent astringent agent to create a new gingival retraction paste. Many studies have shown that this material has many beneficial properties, so it was chosen. It can be applied topically to wounds as an astringent antiseptic, and it aids in the tightening and healing of inflamed mucous membranes. Furthermore, Boswellia papyrifera has low acidity (pH=6), is inexpensive, readily available, and simple to prepare as an aqueous extract [7-9].
The active constituents are Boswellic acids, which are found in the extracted Boswellia terpenoid component (astringent) [10].
Boswellia is a stem exudation; it is an oleo gum resin of the Boswellia genus, which has 17 genera and 600 species and belongs to the Burseraceae family. For centuries, gum has been widely used in medicine to treat various ailments, especially rheumatism and skin diseases [11,12].
In Africa, Boswellia papyrifera is one of those species that provide numerous economic and ecological benefits. Ethiopia, Nigeria, Cameroon, the Central African Republic, Chad, Sudan, Uganda, and Eritrea all have it [13]. The species is widely known for its Frankincense.
This study aimed to fabricate new gingival retraction pastes based on the herbal astringent agent (Boswellia papyrifera) with simple operation, good controllability, low acidity, reasonable price, higher Compatibility with polyvinyl siloxane impression material by measuring the setting time polymerization index and compared them with conventional gingival retraction pastes based on 15%AlCl3 (Astringent Retraction Paste, 3M ESPE, Germany).
Methods and Participants
Preparation of Boswellia papyrifera aqueous extract
The crude of Boswellia papyrifera used in this study was collected from a local traditional market in Baghdad, Iraq, and came from Ethiopia.
The extract has been checked by the Department of Biology at the University of Baghdad, College of Science for Women.
Traditional methods were used to make the aqueous extract [14,15]. The synthesis of experimental gingival retraction pastes samples was done at the basic science, Department of the dentistry college - University of Baghdad.
Preparation of gingival retraction pastes in an experimental setting
The water swelling gingival retraction paste manufacturing method was used in the production of the experimental gingival retraction pastes [16].
Two new gingival retraction pastes based on Bp, with and without AlCl3 (5 % Bp +7.5 % AlCl3) and (5 % Bp), respectively, have been produced. Polyvinylpyrrolidone (PVP), Kaolin, and Deionized Water were also used in the production of both pastes.
Preparation of experimental gingival retraction paste containing (5% Bp +7.5% AlCl3)
In a cold-water bath, 20 ml of 15% AlCl3 aqueous solution was combined with 20 ml of 10% aqueous extract of Bp for 5 minutes in a thermostatic stirrer using a 1 cm para magnetic stirrer bar with a rotational speed of 100r/Second. 1gm PVP powder was then applied to the previous mixture and stirred for 30 minutes in a thermostatic stirrer over a hot water bath (70 oC) until all polymers was dissolved. For the next 30 minutes, 14 g kaolin was added incrementally and slowly with a stirrer until the paste was formed.
Preparation of experimental gingival retraction paste containing (5% Bp)
Under a hot water bath (70 oC) in a thermostatic stirrer with a rotational speed of 100r / second for 30 minutes, 40 ml of 5% aqueous extract of Bp was combined with 1gm powder of PVP until all polymer was dissolved. For the next 30 minutes, 14 g kaolin was added incrementally and slowly with a stirrer until the paste was formed.
PH Measurement
A digital pH meter (Hanna, Mauritius) was used to calculate the pH of the experimental and control gingival retraction pastes after a 1:10 dilution in distilled water at 21°C. The pH of each paste was measured in triplicate, and the mean values were then determined. The pH values for (5% Bp +7.5% AlCl3), (5% Bp), and (3M) were (6.07), (4.5), and (4.43), respectively, according to the results.
The compatibility test
The test of the Compatibility with Light body type III polyvinylsiloxane (PVS) impression material (Variotime light body type III, KULZER, Germany), was carried out by measuring the standard-setting time of polyvinylsiloxane impression material to obtain the ultimate compatibility time polymerization index (100%) for the control group and compared it with the compatibility time polymerization index of the impression after mixing with the studied gingival retraction pastes at oral temperature (35 ± 0.1) °C; using Brookfield HBDVII+ viscometer (USA), this study was performed at University of Technology/ Department of Material Engineering.
Sample grouping
The thirty-two samples were divided into four groups of eight for each according to the type of gingival retraction material that would be mixed with PVS impression material.
Control=PVS impression without mixing with gingival retraction paste.
Group 1=Novel gingival retraction paste (5% Bp+7.5% AlCl3 mixed with PVS impression material.
Group 2=Novel gingival retraction paste (5% Bp) mixed PVS impression material.
Group 3=Conventional gingival retraction paste (Astringent Retraction Paste, 3M ESPE, Germany) mixed with PVS impression material.
Preparation the specimens
The specimens were prepared by mixing (6.6) gm of PVS impression with 40 μl (0.07g for experimental and 0.08 for conventional) gingival retraction pasts in a cylindrical plastic specimen container with dimensions (6.5 cm in height and 3.5 cm in diameter).
While the specimens of control groups were performed without mixing with any gingival retraction pastes, thisstudy was conducted with a viscometer at 0.5 rpm and spindle size (0.7) at oral cavity temperature (35 ± 0.1)°C(Figure 1) [17].
Figure 1: Brookfield HBDVII+ viscometer.
The impression material for each specimen was increased in viscosity until the highest point of torque was reached. The setting time was collected once the impression released by dispensing tip to the highest point of torque (shear force) was reached to achieve the highest viscosity in centipoises (cP).
The compatibility time polymerization index (CTPI) was expressed as 100% for the control group (without contact with any gingival retraction paste) [18].
CTPI = (the setting time in sec. of group contact with gingival retraction paste/ average number of setting time in a sec. of the control group) × 100
Thus if the CTPI was 100% for other groups (contact with gingival retraction pastes) that meant CTPI of the impression was identical to its control and represented the optimum Compatibility; otherwise, if the index value below or higher than 100%, it would be less Compatibility.
Results
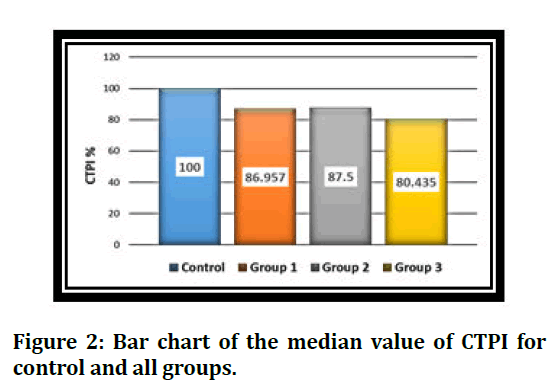
Descriptive statistics (Median, Mean rank, Min., Max.), and inferential statistics, Kruskal Wallis H test (KWH) test, and Mann-Whitney U test (MWU) test for (CTPI) among the groups are illustrated in (Tables 1 and 2, and Figure 2).
Table 1: Descriptive statistics and KWH test of CTPI among the group0073.
| Groups | Descriptive statistics | Comparison | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Median (%) | Mean rank | Min. | Max. | KWH test | p-value | SIG | |
| Control | 8 | 100 | 28.25 | 94.565 | 103.261 | 22.557 | 0 | HS |
| Group 1 | 8 | 86.957 | 16.063 | 86.957 | 95.652 | |||
| Group 2 | 8 | 87.5 | 15.125 | 82.609 | 92.391 | |||
| Group 3 | 8 | 80.435 | 6.563 | 80.435 | 86.957 | |||
Table 2: MWU test of CTPI for multiple comparisons between groups.
| Comparisons between groups | MWU test | p-value (P> 0.05) | Sig. |
|---|---|---|---|
| Control vs. Group 1 | 2 | 0.001 | HS |
| Control vs. Group 2 | 0 | 0.001 | HS |
| Control vs. Group 3 | 0 | 0.001 | HS |
| Group 1 vs. Group 2 | 30 | 0.826 | NS |
| Group 1 vs. Group 3 | 7.5 | 0.005 | HS |
| Group 2 vs. Group 3 | 9 | 0.013 | S |
Figure 2: Bar chart of the median value of CTPI for control and all groups.
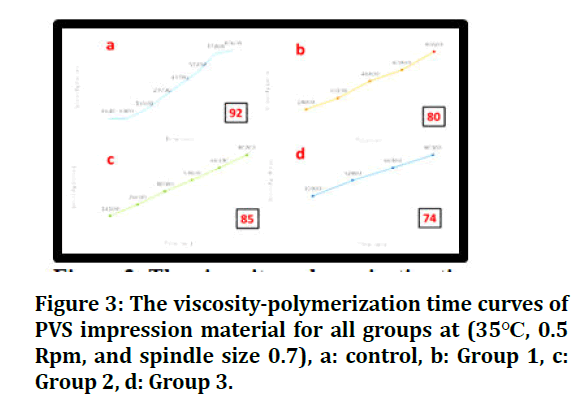
This test revealed that the highest CTPI was seen in experimental gingival retraction paste in group 2 (5% Bp). In contrast, the lowest CTPI was seen in conventional gingival retraction paste, group 3 (3M), with a significant difference between them; their median values were 87.5% and 80.543%, respectively. While there were no significant differences between experimental gingival retraction pastes, group 1 (5% Bp +7.5% AlCl3), and group 2 (5% Bp), the median values were 86.957% and 87.5%, respectively. The viscositypolymerization time curves of PVS impression material for all groups are illustrated in Figure 3 to represent the polymerization time for each group and the rheological behaviour of PVS impression for all groups.
Figure 3: The viscosity-polymerization time curves of PVS impression material for all groups at (35°C, 0.5 Rpm, and spindle size 0.7), a: control, b: Group 1, c: Group 2, d: Group 3.
This Figure shows that the polymerization time of the control group was 92 sec. at 35°C while there were decreasing in polymerization time for other groups (groups 2, group 1, and group 3) that were 85 sec., 80 sec., 74 sec. Respectively at the same temperature.
Discussion
Many types of researches showed some chemical agents might cause an adverse effect on the physicochemical properties or polymerization time of elastomeric impression material. Like Sulphur contamination from latex gloves, latex rubber dam, and some gingival retraction agents like AlCl3, and Ferric Sulphate, then care must be taken to remove all remnants of these medicaments before taking the impression [18-20].
Many authors were encouraged to develop biomaterial gingival retraction pastes with low acidity [6,21,22].
According to the other researches, they have pretended the higher pH of gingival retraction paste could enhance their Compatibility with elastomeric impression materials [20,23].
The compatibility test was carried out in this study by measuring the setting time of PVS impression material before and after mixing with novel gingival retraction pastes based on the herbal astringent agent (Bp) with and without AlCl3 and control gingival retraction paste based on AlCl3.
Danuta Nowakowska et al. showed the rheological study expressed by setting time compatibility test was a sensitive and reliable method for evaluating the Compatibility of PVS after contact with different gingival retraction agents. The optimum CTPI value should be 100% for the control group (the impression without mixing with gingival retraction pastes), indicating full Compatibility [24].
The result of this study showed that the highest CTPI was seen in group 2 (experimental gingival retraction paste based on 5% Bp), its compatibility index was 87.5%. pH was 6.7, followed by group 1(experimental gingival retraction pastes based on 5% Bp +7.5% AlCl3), its compatibility index was 86.95%, and pH was 4.5. In contrast, the lowest Compatibility was seen in group 3 (control), its compatibility index was 80.43%, and pH was 4.43.
However, all these pastes were represented as a high compatible since, according to another study, their authors represented the 80% compatibility index as high Compatibility [24].
This result revealed that the Bp was more compatible with PVS impression than AlCl3, the possible causes might be attributed to low acidity of Bp, this result was agreed with other researches, they pretended the low acidity of gingival retraction paste could enhance their Compatibility with elastomeric impression material [20,23].
Furthermore, the compatibility index of group 1 was more than group 3, even though their acidity was very close between them; this result confirmed that the low concentration of AlCl3 (<10) was more compatible with the PVS impression than the higher concentration (15%). This result was agreed with Danuta Nowakowska et al., who showed that at 37 Cº, The PVS impression in contact with AlCl3 had low tensile strength, and high Compatibility was registered for 10% AlCl3. In contrast, 20% AlCl3 inhibited polymerization [24]. Other studies also preferred using AlCl3 less than 10% [22,25,26].
In contrast, Singh et al. showed an adverse effect of Compatibility when the 5% AlCl3 was in contact with PVS impression material after soaking with retraction cords [27].
While Machado et al. concluded that AlCl3 solutions did not exhibit any inhibitory potential on the addition of silicone, most likely, the visual inspection applied in these studies was not accurate enough to prove the interactions between chemical displacement agents and addition silicone impression material [28]. The same authors showed that two agents (20% and 25% AlCl3) resulted in negative PTCI values [29].
Within limitation of this study, it was concluded that the novel gingival retraction gingival pastes based on low acidity herbal astringent agents (Boswellia paperifera) exhibit higher Compatibility with polyvinylsiloxane impression than the conventional gingival retraction pastes based on the 15% AlCl3.A further suggestion could be recommended to test these novel gingival retraction pastes with other elastomeric impressions such as polyether impression materials.
Conclusion
Within the limitation of this study, it was concluded:
Both novel gingival retraction pastes based on low acidity astringent agents (Boswellia papyrifera) with and without low concentration AlCl3 (<10%) showed higher Compatibility with polyvinylsiloxane impression martial than the conventional gingival retraction paste based on 15% AlCl3.
There was a negative relationship between the acidity of gingival retraction paste and their Compatibility with polyvinylsiloxane impression martial.
References
- Al-Ani A, Bennani V, Chandler NP, et al. New Zealand dentists’ use of gingival retraction techniques for fixed prosthodontics and implants. NZ Dent J 2010; 106:92-96.
- Donovan TE, Chee WW. Current concepts in gingival displacement. Dent Clin 2004; 48:433-444.`
- Yang, J.C T, Lee, H K T, Lee, S. Y. T, et al. European Patent Application. 2006; 1:1–14.
- Chen, X, Qian X. Patent Application Publication. No:US 2008/0225123 A1. 2008.
- Csempesz F, Vág J, Fazekas Á. In vitro kinetic study of absorbency of retraction cords. J prosthet Dent 2003; 89:45-49.
- Mitchell L. Displacement of a mandibular canine following fracture of the mandible. Br Dent J 1993; 174:417-418.
- Eyre H, Hills MJ, Watkins SD. Compositions containing Boswellia extracts. United States patent US 6,589,516. 2003.
- Hamidpour R, Hamidpour S, Hamidpour M, et al. Frankincense (RÇ? XiÄng; Boswellia species): From the selection of traditional applications to the novel phytotherapy for the prevention and treatment of serious diseases. J Tradit Complement Med 2013; 3:221–226.
- Gaikwad VM, Hindole SS. Formulation and evaluation of Boswellia serrata resin gel by using different gelling agents. Int J Bio-Pharma Res 2019; 8:2763-2768.
- Krieglstein CF, Anthoni C, Rijcken EJ, et al. Acetyl-11-keto-β-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int J Colorectal Dis 2001; 16:88-95.
- Alam M, Khan H, Samiullah L et al. A review on phytochemical and pharmacological studies of Kundur (Boswellia serrata Roxb ex Colebr.)-A Unani drug. J Appl Pharm Sci 2012; 2:148-156.
- Sultana A, Padmaja AR. Boswellia serrata Roxb. A traditional herb with versatile pharmacological activity: a review. Int J Pharma Sci Res 2013; 4:2106-2117.
- Vollesen, K. Burseraceae. Flora of Ethiopia 1989; 3:442–478.
- Khalaj-Kondori M, Sadeghi F, Hosseinpourfeizi MA, et al. Boswellia serrata gum resin aqueous extract upregulates BDNF but not CREB expression in adult male rat hippocampus. Turkish J Med Sci 2016; 46:1573-1578.
- Khalaj-Kondori M, HosseinpourFeizi MA, ShaikhzadehHesari F. The effect of aqueous extract of Boswellia on spatial learning and memory in adult male rats. J Adv Med Biomed Res 2014; 22:122-131.
- Salman HA, Majeed MA, AL-Rubaee EA. Evaluation of the cytocompatibility of novel gingival retraction pastes based on boswellia papyrifera. Int J Pharma Res 2021; 13.
- Martins F, Reis J, Barbero Navarro I, et al. Dimensional stability of a preliminary vinyl polysiloxane impression material. Dent J 2019; 7:81.
- Nowakowska D. Classification of chemical retraction agents. Dent Prosthet 2008; 58:202-208.
- O'Mahony A, Spencer P, Williams K, et al. Effect of 3 medicaments on the dimensional accuracy and surface detail reproduction of polyvinyl siloxane impressions. Quintessence Int 2000; 31.
- Sábio S, Franciscone PA, Mondelli J. Effect of conventional and experimental gingival retraction solutions on the tensile strength and inhibition of polymerization of four types of impression materials. J Appl Oral Sci 2008; 16:280-285.
- Moraes Melo Neto CL, Borges HF, Firmino DE Souza Y, et al. Comparison between aluminum chloride and tetryzoline hydrochloride for control of vertical gingival displacement and crevicular fluid. Revista de Odontologia da UNESP 2017; 46:220-226.
- Yang JC, Tsai CM, Chen MS, et al. Clinical study of a newly developed injection-type gingival retraction material. Chin Dent J 2005; 24:147-151.
- Nowakowska D, Saczko J, Kulbacka J, et al. Cytotoxic potential of vasoconstrictor experimental gingival retraction agents-in vitro study on primary human gingival fibroblasts. Folia Biologica 2012; 58:37-43.
- Nowakowska D, Raszewski Z, Saczko J, et al. Polymerization time compatibility index of polyvinyl siloxane impression materials with conventional and experimental gingival margin displacement agents. J Prosthet Dent 2014; 112:168-175.
- Jokstad, A. Clinical trial of gingival retraction cords. J Prosthet Dent 1999; 81:258–261.
- KopaÄ I, Sterle M, Marion L. Electron microscopic analysis of the effects of chemical retraction agents on cultured rat keratinocytes. The J Prosthet Dent 2002; 87:51-56.
- Singh R, Singh J, Gambhir RS, et al. Comparison of the effect of different medicaments on surface reproduction of two commercially available Polyvinyl Siloxane impression materials-An Invitro Study. J Clin Exp Dent 2013; 5:e138.`
- Machado CE, Guedes CG. Effects of sulfur-based hemostatic agents and gingival retraction cords handled with latex gloves on the polymerization of polyvinyl siloxane impression materials. J Appl Oral Sci 2011; 19:628-633.
- Machado CE, Guedes CG. Effects of sulfur-based hemostatic agents and gingival retraction cords handled with latex gloves on the polymerization of polyvinyl siloxane impression materials. J Appl Oral Sci 2011; 19:628-633.
Author Info
Hiba A Salman1*, Manhal A Majeed2 and Eaman A Al-Rubaee1
1Department of restorative and aesthetic dentistry, College of Dentistry, Baghdad University, Iraq2Department of Basic Science, College of Dentistry, Iraq
Citation: Hiba A Salman, Manhal A Majeed, Eaman A Al-Rubaee,Compatibility of Novel Gingival Retraction Pastes Based on Boswellia papyrifera with Polyvinyl Siloxane Impression Material (A Comparative in-vitro Study), J Res Med Dent Sci, 2021, 9(7): 127-132
Received: 05-Jun-2021 Accepted: 08-Jul-2021