Research - (2021) Volume 9, Issue 5
Complications accompanying lateral Sinus Augmentation
Ali H Abbas Alhussaini1* and Thair A Lateef Hassan2
*Correspondence: Ali H Abbas Alhussaini, Department of Oral and Maxillofacial Surgery, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: The most common intraoperative complications of lateral sinus augmentation are hemorrhage and Schneiderian membrane perforation. Aims: To evaluate the incidence of complications during lateral sinus augmentation surgery, and to estimate the potential risk factors. Materials and Methods: Twenty-five patients, ages ranged from 25-72 years, met the eligibility criteria. A total of 35 lateral sinus augmentation procedures were performed utilizing demineralized bovine bone and 52 dental implants simultaneously installed. The potential risk factors were evaluated clinically and radiographically, to analyze their relationship with the incidence of intra and postoperative complications. Results: The alveolar antral artery was detected by cone beam computed tomography scans in 30 cases (85.71%) out of 35 lateral sinus augmentation procedures. Intraoperative hemorrhage occurred in 8 cases (26.67%). There was a significant correlation between vessel diameter ≥ 1 mm and the occurrence of hemorrhage (P=0.0001). The incidence of Schneiderian membrane perforation was 11 (31.42 %). There was no statistically significant correlation between patient, surgery, and maxillary sinus properties-related risk factors with intraoperative membrane perforation, except one significant risk factor which was the alveolar antral artery with a diameter ≥ 1 mm. Conclusions: The present study deduced that alveolar antral artery with a diameter ≥ 1 mm is the only risk factor that statistically significantly increased the incidence of hemorrhage and Schneiderian membrane perforation during lateral sinus augmentation procedure.
Keywords
CBCT, Complications, Lateral sinus augmentation, Sinus membrane perforation
Introduction
Maxillary sinus augmentation is a well-accepted procedure in the atrophic posterior maxilla, utilizing various grafting materials for achieving adequate bone height and volume suitable for installation of dental implants (DI). Several techniques have been used, including lateral approach and transcrestal approach. The lateral sinus augmentation (LSA) was reported by Tatum in the mid-seventies and published for the first time by Boyne and James in 1980 [1].
Nevertheless, LSA might associate with certain intraoperative and postoperative complications that may influence the outcome of the therapy. The most common intraoperative complication that occurs during LSA surgery is sinus membrane perforation (SMP) [2]. There are many potential risk factors influencing the occurrence of SMP including: thin sinus membrane, presence of sinus septa, residual bone height (RBH) Ë?3 mm, narrow sinus width, thick sinus lateral wall, irregular sinus floor, previous surgery, sinus pathology, overfilling with the graft material, bounded saddle with single missing tooth, and limited mouth opening [3,4]. Sindel et al. [5] declared that a thorough knowledge of possible risk factors and proper management of these complications are essential to obtain better treatment outcomes.
The second most common complication during lateral window preparation is hemorrhage. Maridati et al. [6] stated that the transection of intraosseous alveolar antral artery (AAA) with a diameter over 2mm may result in hemorrhage and impairment of vision, which may lead to a potential membrane perforation.
Several studies evaluated multiple potential risk factors to aid surgeons in performing a comprehensive presurgical evaluation prior to LSA procedure [4,7].
Major postoperative complications are relatively uncommon, which include postoperative graft infections, maxillary sinusitis, oroantral fistula, loss of graft material, maxillary cyst formation, migration of dental implants into the sinus cavity proper, and failure of DI [8].
The aims of this study were to evaluate the prevalence of hemorrhage and SMP, and to estimate the correlation between potential risk factors and the occurrence of complications during LSA surgery.
Materials and Methods
Study sample
This randomized prospective clinical study was conducted at the Dental Implant Unit/ Department of Oral and Maxillofacial Surgery/ College of Dentistry/University of Baghdad, from January 2019 to August 2020. Twenty-five patients (15 females and 10 males), with age ranged from 25-72 years (mean of 51.5 years) with atrophic edentulous posterior maxillary region were selected according to the eligibility criteria for LSA procedures following a thorough preoperative dental and medical history, clinical and radiographic examinations.
The inclusion criteria included: healthy individuals without any systemic disease/local pathological lesion at the sinus zone, patient’s age ≥ 18 years, the RBH was ≥ 3 ≤ 6 mm with adequate quantity and quality of native bone to gain primary implant stability for simultaneously installed DI, and healed implant insertion site at least 6 months after tooth extraction, and smokers (< 20 cigarettes per day). A total of 35 LSA procedures were accomplished utilizing demineralized bovine bone and 52 DI installed simultaneously.
The project of the study was approved by the Ethical Committee of the College of Dentistry/ University of Baghdad (reference no. 35 in 9/1/2019). All patients were informed about the nature of the study and they signed a written consent form for their participation in this study.
Radiological examination
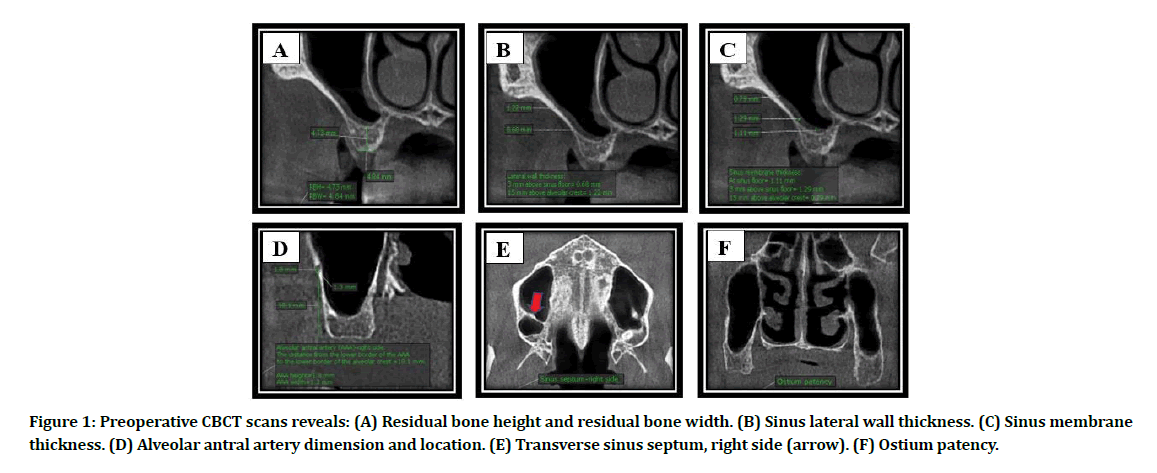
Panoramic radiograph was obtained preoperatively for preliminary evaluation of the maxillary sinus (MS). Cone beam computed tomography (CBCT) scan was recommended when the patient elected for LSA to provide a precise preoperative evaluation of RBH and residual bone width (RBW) at the planned implant recipient site, sinus lateral wall thickness (LWT) at 3 mm above sinus floor and 15 mm above alveolar crest, sinus membrane thickness (SMT) at sinus floor, 3 mm above sinus floor and 15 mm above alveolar crest, the diameter of the AAA and the distance from its inferior border to the alveolar crest, presence and orientation of sinus septum, ostium patency, (Figure 1A-Figure 1F). Additional CBCT scans were obtained at 2 weeks and 24 weeks after surgery to evaluate the graft healing and bone-implant contact.
Figure 1: Preoperative CBCT scans reveals: (A) Residual bone height and residual bone width. (B) Sinus lateral wall thickness. (C) Sinus membrane thickness. (D) Alveolar antral artery dimension and location. (E) Transverse sinus septum, right side (arrow). (F) Ostium patency.
Surgical procedure
All surgical procedures were accomplished by an experienced surgeon with this sort of procedures. Patients were premedicated with 400 mg of Cefixime 1 hour prior to surgery.
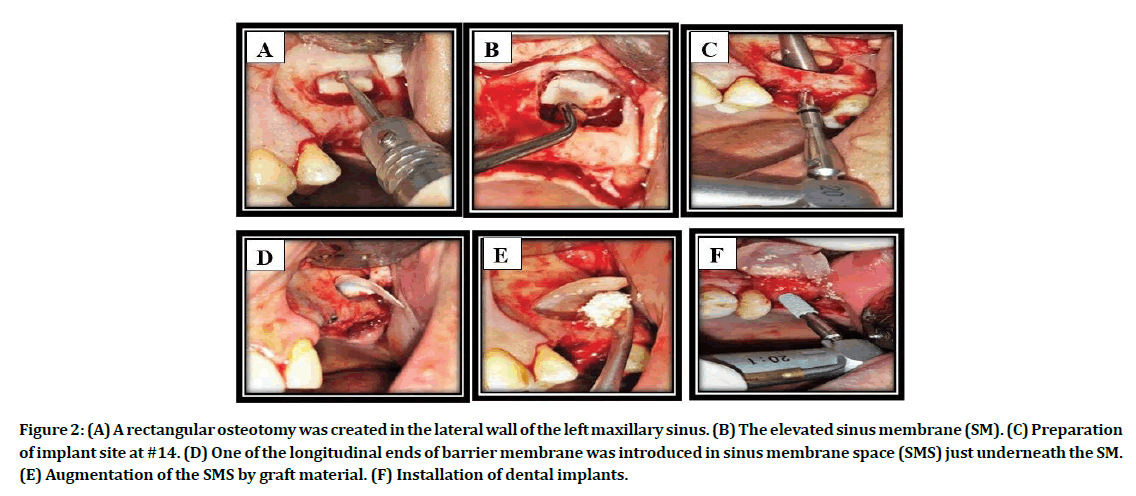
Under local anesthesia (lidocaine 2% with adrenaline 1:80,000, Septodont, France), a full thickness mucoperiosteal flap was performed and reflected to expose the alveolar ridge and the lateral wall of the MS. Lateral window osteotomy was performed, using a conventional drilling technique (round carbide and diamond burs) with copious cooled normal saline irrigation (Figure 2A). Gentle elevation of the sinus membrane with the attached bony island and pushed them inward and upward toward the medial sinus wall utilizing Frios Sinus Set elevators (Dentsply Friadent, Germany), as in Figure 2B. Checking the sinus membrane integrity at this stage by asking the patient to take deep breath.
Figure 2: (A) A rectangular osteotomy was created in the lateral wall of the left maxillary sinus. (B) The elevated sinus membrane (SM). (C) Preparation of implant site at #14. (D) One of the longitudinal ends of barrier membrane was introduced in sinus membrane space (SMS) just underneath the SM. (E) Augmentation of the SMS by graft material. (F) Installation of dental implants.
Preparation of implant insertion sites using NucleOss T6 surgical kit (Turkey) was done with care to prevent SMP when the drill passes through sinus floor, a mucoperiosteal elevator could be helpful to maintain the membrane high and far from the drill in order to avoid this complication (Figure 2C). In all cases, barrier membrane was applied (Figure 2D). Augmentation of the created space with deproteinized bovine bone (BEGO OSS, mebios GmbH, Germany), as demonstrated in Figure 2E. Installation of the DI (NucleOss T6, Turkey) into the prepared osteotomy site (Figure 2F). Wound closure was accomplished using 3/0 black silk suture.
Postoperative examination
Two weeks after surgery, suture removal and evaluate the wound healing. Twenty-four weeks postoperatively, clinical examination of the implanted area was performed to observe if there is exposed cover screw, peri-implantitis, or implant mobility.
Statistical analysis
The Chi Square test was used in this research for statistical analysis of complications and its relationship with possible risk factors. The significant difference was considered when P ≤ 0.05. All the data were analyzed using SPSS version 24.
Results
Twenty-five patients (15 males, 20 females) were included in this study with a total of 35 LSA procedures and 52 DI simultaneously installed (one-stage technique). Mean age of the patients was 51.3 ±12.6 years s (ranging from 25 to 72 years). The mean RBH was 4.87 ± 1.04 mm with a range of (3-6 mm) and the mean RBW was 5.45 ±1.12 mm with a range of (5-9 mm). The total mean LWT was 1.09 ± 0.43 mm. The mean LWT were 1.10 ±0.52 mm and 1.08 ± 0.43 mm at 3 mm above sinus floor and 15 mm above alveolar crest, respectively. The total mean SMT was 2 ± 1.5 mm. The mean SMT were 2.38 ± 1.85 mm, 2.22 ±1.74 mm and 1.41 ±1.36 mm, at sinus floor, 3 mm above sinus floor and 15 mm above alveolar crest, respectively.
Of the 35 MS included a total of 30 cases (85.71 %) in which AAA revealed by CBCT at the surgical field. The mean diameter of AAA was 0.88 ±0.54 mm (ranged 0.3-2 mm). Twenty AAA (66.67%) with a diameter <1 mm, which is twice in number when compared to the diameter ≥ 1mm (33.33%), as revealed in Table 1. The mean distance from the inferior border of AAA to the alveolar crest was 14.18 ±4.01mm (ranged 7-20 mm). Thirty-three LSA procedures (94.29%) were performed in molar region with 47 DI (90.38%).
Table 1: Diameter of alveolar antral artery and the incidence of hemorrhage.
| Alveolar Antral Artery | ||||
|---|---|---|---|---|
| Diameter (mm) | Revealed by CBCT No. (%) |
Presence of hemorrhage clinically No. | P-value* | |
| Yes No. (%) |
No No. (%) |
|||
| < 1 | 20 (66.67) | 1 (5) | 19 (95) | 0.0001 |
| ≥ 1 | 10 (33.33) | 7 (70) | 3 (30) | |
| Total (%) | 30 (100) | 8 (26.67) | 22 (73.33) | |
*, Chi-Square test; No., number; %, percentage.
Complications
Intraoperative complications
Hemorrhage
Out of 30 AAA revealed by CBCT, 8 cases (26.67%) suffered from intraoperative hemorrhage, 7 cases (70%) of which occurs in AAA with a diameter ≥ 1mm. A highly significant positive correlation manifested between AAA diameter ≥ 1mm and the occurrence of hemorrhage at the surgical field (P= 0.0001). No statistically significant correlation evident between the location of AAA and the coincidence of hemorrhage at the surgical field (Table 2).
Table 2: Location of alveolar antral artery and the incidence of hemorrhage.
| Alveolar Antral Artery | ||||
|---|---|---|---|---|
| Distance from the alveolar crest (mm) | Revealed by CBCT No. (%) |
Hemorrhage | P-value* | |
| Present No. (%) |
Absent No. (%) |
|||
| 7-13 | 13 (43.33) | 4 (30.77) | 9 (69.23) | 0.698 |
| 14-20 | 17 (56.67) | 4 (23.53) | 13 (76.47) | |
| Total (%) | 30 (100) | 8 (26.67) | 22 (73.33) | |
*,Chi-Square test; CBCT, cone beam computed tomography.
Sinus membrane perforation
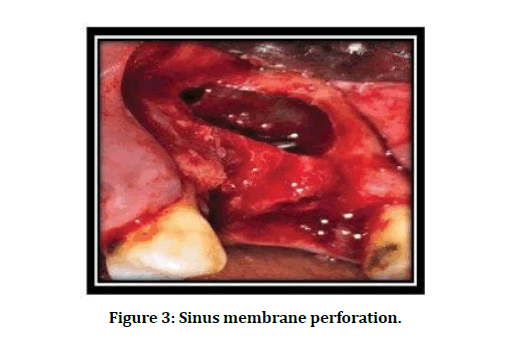
The overall incidence of SMP was 11 (31.42 %) out of total 35 LSA procedures. Seven SMP (63.64%) observed with a size of > 2 < 5 mm. Most SMP (90.91%) occurred at the upper border of the lateral window (class I), as illustrated in Figure 3 and Table 3.
Figure 3: Sinus membrane perforation.
Table 3: Distribution of sinus membrane perforation according to its size and location.
| Sinus membrane perforation size (mm) |
Sinus membrane perforation class | Total | |||
|---|---|---|---|---|---|
| I | II | III | |||
| Sinus membrane perforation No. (%) | No. | % | |||
| Total | 10 (90.91) | 1 (9.09) | 0 (0) | 11 | 100 |
| ≤ 2 | 4 (100) | 0 (0) | 0 (0) | 4 | 36.36 |
| > 2 < 5 | 6 (85.71) | 1 (14.29) | 0 (0) | 7 | 63.64 |
*, Chi-Square test, LSA, lateral sinus augmentation; SMP, sinus membrane perforation.
Possible risk factors of sinus membrane perforation
The three main risk factors that influence the incidence of SMP are:
Patient-related factors
Table 4 demonstrates that there was no statistically significant association between patient-related risk factors (gender, age, and smoking habit) with the incidence of SMP (p>0.05). However, patients’ age ≥ 40 years suffered SMP more than two times as often (35.71%) as aged <40 years (14.28%), but this difference was not statistically significant (P =0.392).
Surgery-related factors
Table 5 presents that surgery-related risk factors (side of lateral window, number of inserted implants, DI inserted region, type of edentulism relative to the maxillary sinus, and the enucleation of mucocele) did not significantly influence the rate of SMP.
Maxillary sinus properties-related factors
Table 6 reveals that RBH, LWT, and SMT did not associated with a significant difference in the prevalence of SMP. In contrast, AAA with a diameter ≥ 1 mm influence SMP six times as often (60%) as < 1mm (10%). A significant positive correlation manifested between AAA diameter ≥ 1mm and the occurrence of SMP (P= 0.007).
Postoperative complications
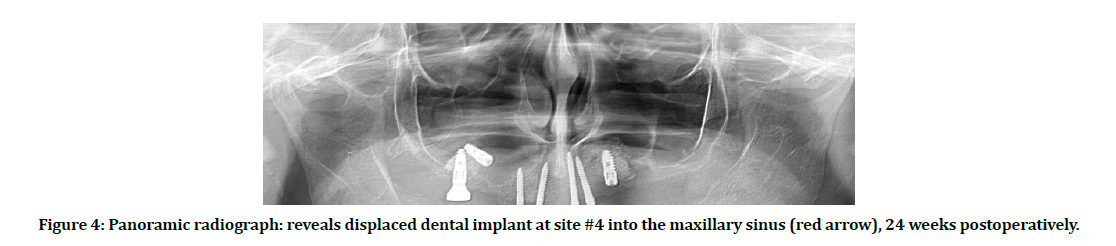
Soft tissue dehiscence with exposed cover screws presented in 3 DI (5.77%), peri-implantitis manifested in 2 adjacent DI (3.85%), and failure occurred in 2 adjacent DI (3.85%), one of these fixtures displaced into the MS (Figure 4).
Figure 4: Panoramic radiograph: reveals displaced dental implant at site #4 into the maxillary sinus (red arrow), 24 weeks postoperatively.
Discussion
Hemorrhage
The incidence of hemorrhage in this study (26.67%) is higher than that mentioned by Saad et al. [9] who stated that intraoperative bleeding from injured AAA was 15.79% during lateral window preparation. This might be related to the differences in diameter and position of AAA in both studies. However, those authors did not mention about these variables in their study.
The intraosseous AAA manifested by CBCT scans in 30 MS (85.71%) out of 35 MS, which is higher in comparison to other study in which it was observed in 69.6% from 860 MS utilizing CBCT scans [10]. The difference between the studies might be related to small sample size of the current research.
The mean vascular diameter was 0.88 ±0.54 mm (ranged 0.3-2 mm). This result was close to the outcome mentioned by Danesh-Sani and coworkers 2017 [10] who stated that the mean diameter of the AAA was 1.17 mm (range 0.4- 2.8 mm). The current survey observed a highly significant positive correlation present between AAA diameter and the occurrence of hemorrhage during lateral window preparation (P =0.0001).
Most of the hemorrhage cases took place for AAA ≥ 1mm in diameter. The explication for arterial injury might be related to the diameter and position of the AAA intervened during lateral antrostomy preparation which in most cases was unavoidable according to the requested location and size of the lateral window. This outcome was supported by other study which achieved a higher incidence of bleeding when the AAA diameter > 2 mm [11].
The mean distance of the AAA away from the alveolar crest was 14.18 ±4.01 mm (ranged 7-20 mm). This result is in agreement with other studies which found that the mean distance of AAA from the residual alveolar crest in the first molar area varies from 14.9 to 16.96 mm, measured using CBCT scans [12,13].
The hemorrhage controlled by the same method mentioned by Kim and Jang in 2019 [14], through using a high-speed handpiece installed with a diamond bur and applied to the bleeding point without irrigation for cauterization of the severed vessel which was quite successful in all cases for salvage of this problem.
Sinus membrane perforation
The overall incidence of SMP (31.42 %) in the current study was within the range reported in the literatures that occurred during lateral window approach which ranged from 11.5% to as high as 56% [2,15,16].
All SMP occurred during preparation of the lateral window using conventional rotary instruments. Jordi et al. 2018 [17] reported in their meta-analysis study, that conventional rotary instruments were associated with a high perforation rate (24%) in comparison to the piezoelectric devices (8%) for the preparation of the maxillary lateral wall, with statistically significant difference between both techniques.
Seven SMP (63.64 %) out of 11 membrane perforations were existed with a perforation size > 2 < 5 mm. Most of the SMP (90.91%) occurred at the upper border of the prepared lateral window (class I). Fugazzotto and coworkers in 2015 [18] stated that the presence of a class I SMP poses no concerns with regard to either sequencing of therapy or the final treatment result, assuming appropriate perforation management.
Possible risk factors of sinus membrane perforation
Several authors suggested that three main risk factors might influence the incidence of SMP [4,7,19].
Patient-related factors
There was no statistically significant association between patient-related risk factors (gender, age, and smoking habit) with the incidence of SMP (p > 0.05). Similarly, Von Arx et al. 2014 [19] published in their retrospective study that the statistical analysis did not show a significant relationship between occurrence of SMP and patient-related risk factors.
According to author’s observation, the other predisposing factors for SMP might be related to uncooperative patient, awareness of the assistant, bulky cheek, and limited mouth opening.
Surgery-related factors
These factors included: side of lateral window preparation, number of inserted implants, DI inserted site, type of edentulism relative to the maxillary sinus, intraoperative hemorrhage, and the enucleation of mucocele. There was no association with the occurrence of SMP from statistical point of view (p > 0.05). This comes in line with other studies which declared that no statistically significant relation present between the above-mentioned variables and the incidence of SMP [4,19].
Maxillary sinus properties related factors
More incidence of SMP associated with RBH Ë?4 mm (33.33%) vs. RBH ≤ 4 mm (25%). However, it did not reach the statistically significant level. This result comes with the line of Tükel et al. [20] who found an increase in SMP during sinus lift with RBH of 3-6 mm when compared with RBH < 3 mm. The author explained this phenomenon by the fact that in sinuses with a lower RBH, the floor of the sinus moves closer to the alveolar ridge, thus making access to the sinus cavity and the manipulation of the instruments easier.
In the existing study, the mean lateral wall thickness (LWT) was 1.09 ±0.43 mm at four standardized measuring points. The LWT did not influence the occurrence of SMP from a statistical point of view. This result is supported by other studies, who found that the thickness of the LSW did not have a bearing on SMP rate [4,19].
In the current study, the total mean SMT at six standardized measuring points was 2.03 ±1.5 mm. The SMT presented in this study was ≤ 2 mm in 57.14% of the total LSA cases and > 2 mm in 42.86%. Lozano-Carrascal et al. [21] found that the mean SMT was 1.82 ± 1.59 mm at the base of the sinus in the 1st molar area. Tavelli et al. [3] reported in their systematic review that normal membrane thickness ranged from 0.8 to 1.49 mm.
There was a high correlation between SMP and SMT. It occurred two folds more in SMT ≤ 2 mm than in SMT Ë? 2 mm. However, it did not reach the statistically significant level (P=0.281). A study deduced after analyzing two-hundred CBCT images, that a membrane thickness ≤ 2 mm may be an important determinant of perforation, regardless of the surgical procedure [22]. Moreover, several studies concluded that the SMP rate was highest when the SMT was <1mm or >3 mm [3,7].
In the present study, the AAA diameter ≥ 1 mm had a statistically significant association with SMP (P= 0.007) in comparison to AAA diameter Ë? 1 mm. This result is supported by Testori et al. 2020 [7] who mentioned that a high risk of SMP occurred when the AAA diameter was > 2 mm. Marin et al. 2019 [4] declared that transecting this vessel can cause minor to severe bleeding that may obscure vision and lead to SMP.
The management of SMP was accomplished by folding the membrane upon itself, since the size of SMP were < 5 mm, followed by the application of collagen barrier membrane which were quite efficient and the wound healing was mostly uneventful, without any undesirable postoperative consequences.
Postoperative complications
Wound dehiscence
Local wound dehiscence and exposed cover screws occurred in 3 patients (5.77%) in this study. This can be attributed to contamination of the wound owing to the dereliction of the patient to preserve good oral hygiene.
Conservative management including; instruction and motivation for good oral hygiene, Prescribing antibiotic for 5 days, chlorhexidine rinses twice a day, permitting for healing by secondary intention.
Peri-implantitis
The total peri-implantitis occurred in two adjacent fixtures (3.85%), for the same patient. On clinical examination, after 44 weeks after DI installation (5 months after fixing gingival formers, postponed the prosthetic rehabilitation since the patient had submitted to bilateral LSA procedures at different periods). The patient had poor oral hygiene and neglected brushing of the gingival formers in which plaque was accumulated on their surfaces. However, these fixtures were stable, and no mobility presented. Robertson et al. [23] reported that peri-implantitis related risk factors are: diabetes mellitus, occlusal overload, smoking, periodontal disease, and poor plaque control.
The management was performed by surgical exploration and augmentation of the deficient area with deproteinized bovine bone and platelet-rich fibrin membrane. The surgical intervention used in this study for treatment of peri-implantitis was done according to the cumulative interceptive supportive treatment protocol D, mentioned by Robertson et al. [23].
Dental implant failure
Two adjacent DI failed for one patient, making the overall failure rate (3.85%). One of these failed implants at #4 site displaced into the maxillary sinus cavity. In second-stage surgery after a healing period of 24 weeks, during excision of the soft tissue covering the DI for measuring the secondary stability, the fixture displaced accidentally into the MS. Panoramic radiograph obtained immediately after this complication to evaluate the condition (figure 4). The other fixture at site #3 was mobile 28 weeks after installation and fall in the patient’s mouth.
The predisposing factors for implant failure in this study came in line with other studies concerning critical factors for early implant failure. Several studies declared that the main causes for early failures during the osseointegration process are: Poor quality and quantity of bone, patient medical condition that affects normal bone healing, unfavorable patient habits, inadequate surgical and prosthetic analysis, biocompatibility of the implant material, suboptimal implant design and surface characteristics [24-26]. Galindo-Moreno et al. 2012 [27] reported that the incidence of implant migration into the sinus cavity is higher for narrower implants, low-density bone, RBH between 5 and 6.9 mm, and in patients with lack of primary implant stability. The management of displaced DI carried out by retrieval of the failed DI utilizing a modified Caldwell-Luc approach.
Conclusion
The present study demonstrates that alveolar antral artery with a diameter ≥ 1 mm is the only factor that significantly increased the risk of hemorrhage and sinus membrane perforation during LSA procedure.
References
- Lozada JL, Goodacre C, Al-Ardah AJ, Garbacea A. Lateral and crestal bone planning antrostomy: A simplified surgical procedure to reduce the incidence of membrane perforation during maxillary sinus augmentation procedures. J Prosthet Dent 2011; 105:147-153.
- Hermes M, Lommen J, Kübler N, Lytvyniuk I, Singh D, Schorn L et al. Influence of Schneiderian membrane perforations on the prognosis and outcomes of lateral window sinus lift operations: A retrospective case series study. J Dent Oral Disord Ther 2018; 6(2):1-9.
- Tavelli L, Borgonovo AE, RE D, Maiorana C. Sinus presurgical evaluation: A literature review and a new classification proposal. Minerva Stomatologica 2017; 66(3):115-31.
- Marin S, Kirnbauer B, Rugani P, Payer M, Jakse N. Potential risk factors for maxillary sinus membrane perforation and treatment outcome analysis. Clin Implant Dent Relat Res. 2019; 21:66–72.
- Sindel A, Özarslan M, Özalp Ö. Management of the complications of maxillary sinus augmentation. 2018, chapter 2. p.27-46 http://dx.doi.org/10.5772/ intechopen.80603.
- Maridati P, Stoffella E, Speroni S, Cicciu M, Maiorana C. Alveolar antral artery isolation during sinus lift procedure with the double window technique. Open Dent J 2014; 30 (8):95-103.
- Testori T, Tavelli L, Yu SH, Scaini R, Darnahal A, Wallace SS, et al. Maxillary sinus elevation difficulty score with lateral wall technique. Int J Oral Maxillofac Implants. 2020; 35(3):631-638.
- Wallace SS, Testori T. Complications in lateral window sinus elevation surgery. In: Froum SJ, editor. Dental implant complications: Etiology, prevention, and treatment. Second edition. John Wiley & Sons, Inc., Hoboken, New Jersey 2016, p. 396-426.
- Saad D, Lateef TA, Hassan AF, Mohammed MQ. The Effectiveness of Platelet Rich Fibrin as a Graft Material in Sinus Augmentation Procedures through Lateral Approach. J. Pharm. Sci. & Res 2018; 10(6):1433-1437.
- Danesh-Sani SA, Movahed A, ElChaar ES, Chan KC, Amintavakoli N. Radiographic evaluation of maxillary sinus lateral wall and posterior superior alveolar artery anatomy: A cone-beam computed tomographic study. Clin Implant Dent and Relat Res 2017; 19(1):151-160.
- Tourbah B, Maarek H. Complications of Maxillary Sinus Bone Augmentation: Prevention and management. In Younes R, Nader N, Khoury G, Editors. Sinus Grafting Techniques: A Step-by-Step Guide. Springer International Publishing Switzerland; 2015. P.195-233.
- Cruz ILA, Palacios VDE, Miranda VJE, Cazar AM, Martínez OPA. CBCT evaluation of the alveolar antral artery in a mexican cohort and its relationship to maxillary sinus floor lifting. Revista ADM 2016; 73 (6): 286-290.
- Varelaâ??Centelles P, Loira M, Gonzálezâ??Mosquera A, Romeroâ??Mendez A, Seoane J, Garcíaâ??Pola MJ, et al. Study of factors influencing preoperative detection of alveolar antral artery by CBCT in sinus floor elevation. Scientific Reports 2020; 10(1):10820.
- Kim J, Jang H. A review of complications of maxillary sinus augmentation and available treatment methods. J Korean Assoc Oral Maxillofac Surg 2019; 45(4):220-224.
- Kasabah S, Krug J, Simunek A, Lecaro MC. Can we predict maxillary sinus mucosa perforation? Acta Med (Hradec Kralove) 2003; 46(1):19-23.
- Malkinson S, Irinakis T. The influence of interfering septa on the incidence of Schneiderian membrane perforations during maxillary sinus elevation surgery: a retrospective study of 52 consecutive lateral window procedures. Oral Surgery 2009; 2(1):19-25.
- Jordi C, Mukaddam K, Lambrecht JT, Kühl S. Membrane perforation rate in lateral maxillary sinus floor augmentation using conventional rotating instruments and piezoelectric device-a meta-analysis. Int J Implant Dent 2018; 4 (1):1-9.
- Fugazzotto P, Melnick PR, Al-Sabbagh M. Complications when augmenting the posterior maxilla. Dent Clin North Am 2015; 59(1):97-130.
- Von Arx T, Fodich I, Bornstein MM, Jensen SS. Perforation of the sinus membrane during sinus floor elevation: A retrospective study of frequency and possible risk factors. Int J Oral Maxillofac Impl, 2014; 29(3):718-726.
- Tükel HC, Tatli U. Risk factors and clinical outcomes of sinus membrane perforation during lateral window sinus lifting: analysis of 120 patients. Int J Oral Maxillofac Surg 2018; 47(9):1189-1194.
- Lozano-Carrascal N , Salomó-Coll O, Gehrke SA, Calvo-Guirado JL , Hernández-Alfaro F , Gargallo-Albiol J. Radiological evaluation of maxillary sinus anatomy: A cross-sectional study of 300 patients. Ann Anat 2017; 214:1-8.
- Rapani M, Rapani C, Ricci L. Schneider membrane thickness classification evaluated by cone beam computed tomography and its importance in the predictability of perforation. Retrospective analysis of 200 patients. Br J Oral Maxillofac 2016; 54(10):1106-1110.
- Robertson K, Shahbazian T, MacLeod S. Treatment of Peri-Implantitis and the Failing Implant. Dent Clin N Am 2015; 59: 329-343.
- Moy PK, Medina D, Shetty V, Aghaloo TL. Dental implant failure rates and associated risk factors. Int J Oral Maxillofac Implants 2005; 20(4):569-77.
- Tolstunov L. Dental implant success-failure analysis: A concept of implant vulnerability. Implant Dent 2006; 9:341-346.
- Turkyilmaz I, Tözüm TF, Tumer C. Bone density assessments of oral implant sites using computerized tomography. J Oral Rehabil 2007; 34(4); 267-272.
- Galindo-Moreno P, Padial-Molina M, Avila G, Rios HF, Hernández-Cortés P, Wang HL. Complications associated with implant migration into the maxillary sinus cavity. Clin. Oral Impl. Res 2012; 23:1152-1160.
Author Info
Ali H Abbas Alhussaini1* and Thair A Lateef Hassan2
1Department of Oral and Maxillofacial Surgery, College of Dentistry, University of Baghdad, Iraq2President of the Iraqi Scientific Council of Maxillofacial Surgery in Iraqi Board for Medical Specializtion, Iraq
Received: 20-Apr-2021 Accepted: 06-May-2021 Published: 13-May-2021