Review Article - (2022) Volume 10, Issue 9
Consequences of COVID-19 in Sickle Cell Disease Patients
Tanvi Punj and Sarika Dakhode*
*Correspondence: Sarika Dakhode, Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (Demmed to be University) Sawangi (Meghe) Wardha, Maharashtra, India, Email:
Abstract
Coronavirus comes from a viral family of Coronaviridae and causes respiratory illness in humans. The symptoms of the disease have shown to range from patients being asymptomatic to some having a mild degree cold and cough to severe pneumonia and thromboembolism which have proven to be fatal for few. Severe illness is however seen mostly in individuals who are either immunocompromised or have an existing comorbidity.
Sickle cell disease is one such comorbidity. COVID-19 has posed unimaginable challenges for health care workers due to its unpredictable effect on various individuals. It is therefore difficult to generalize the disease and the treatment course for those with a known chronic prevalent disorder, such as sickle cell disease. COVID-19 has shown different outcomes and complication in adult and pediatric sickle cell disease patients, making it necessary for us to be prepared with the various complications possible and their treatment modalities.
For this article, we have reviewed articles published till 24 November 2021, by searching on platforms like PUBMED, Google Scholar and EMBASE. We have included all the relevant articles including the incidence, pathophysiology, symptomatology, complications, investigations, treatment modalities and prognosis in patients suffering from sickle cell disease and coronavirus infection.
Keywords
Coronavirus, COVID-19, Sickle cell disease, Thromboembolism, Immunocompromised
Introduction
COVID-19 has shown symptoms ranging from patients being asymptomatic to some having a mild degree cold and cough to severe pneumonia and thromboembolism which have proven to be fatal for few. COVID-19 based on recent studies has shown to be spread by droplet infection, showing major symptoms like fever and cough, anosmia, ageusia, depletion of oxygen saturation and diarrhoea. Severe illness is however seen mostly in individuals who are either immunocompromised or have an existing comorbidity. Several risk factors have been identified for adverse outcomes like age above 65 years, comorbidities like diabetes, chronic illnesses and cardiovascular disorders [1].
Sickle cell disease, commonest hemoglobinopathy recorded globally, has been recognised as a serious health concern by the WHO (World Health Organisation), estimating that sickle cell trait genes constitute about 5% of the global population [2]. The disease is characterised by structural abnormality of normal Haemoglobin (Hb–AA) leading to formation of sickle haemoglobin that is HbS. HbS or Haemoglobin S results from the abnormal folding of beta globin chain due to substitution of valine by glutamic acid at the sixth position. It is characterised by haemoglobin polymerisation, due to low oxygen saturation, which leads to red blood cells to change their morphological shape from being biconcave to a sickle shape, with a change in consistency to rigid sickle shaped cells that clump easily and increase the risk of vasoocclusion, contributing to a higher chance of acute chest syndrome in these patients [3].
Sickle cell trait usually shows normal haematological parameters as HbA attenuates the HbS phenotype, thereby reducing its ability to polymerise [4].
Literature Review
However, the cumulative evidence points that hypoxia is responsible for the various complications like thromboembolism (seen most commonly in pulmonary artery), papillary necrosis of kidney, splenic infarction, rhabdomyolysis and death in sickle cell trait [5].
The World Health Organisation identified COVID-19 as an international public health emergency on 30 January 2020 and declared it a pandemic on 11 March 2020. Coronavirus infection displays a range of symptoms, the severity of infection is determined using the established criteria below [6]:
• Asymptomatic: Positive RT-PCR through nasopharyngeal and oral swabs, absence of any clinical signs and symptoms.
• Mild: General viremia/upper respiratory tract illness, mild grade fever, myalgia, cough, cold, runny nose.
• Moderate: Pneumonia that is positive lung tissue inflammation with or without symptoms, no decrease in oxygen saturation or hypoxia.
• Severe: Early symptomatic respiratory illness, gastrointestinal manifestations, falls in oxygen saturation up to 92% on room air.
• Critical: Respiratory failure, acute respiratory distress, shock, coagulopathy, encephalopathy, multi organ dysfunction leading to multi organ failure (including lung, heart, kidney, brain) [6].
Patients of the benign hemoglobinopathy, sickle cell disease, have been recognized by the centre of disease control and prevention, to be at a much higher susceptibility of suffering severe complications, if infected with the COVID-19 virus [7]. The following are the reasons as to why SCD patients are feared to have a higher susceptibility of severe infection:
• Auto infarction or a prophylactic splenectomy, causes these patients be immunocompromised and hence puts them at a higher risk of developing infectious diseases precipitating into an ACS.
• Pathophysiological hyper inflammatory state leads to an increased risk of thrombus formation.
• Have an increased risk of organ dysfunction secondary to thrombus formation [8].
Discussion
Sickle cell disease epidemiology
According to studies, approximately 5% of the global population has been reported to be a carrier for hemoglobinopathies and have estimated that the Saharan African region is burdened with 2/3rd of the global percentage. The number of new borns with sickle cell disease worldwide as estimated in 2016 was 3,12,000. In India, within a span of 54 years, based on various epidemiological research surveys and in-hospital conducted surveys, it has been found that the prevalence of sickle gene is 1-40% in southern India, 0-18% in north-eastern India, 0-33.5% in western India and 22.5-44.4% in central India. The gene frequency of the HbS variety has been recorded between 0.031-0.041 [9].
Sickle cell and COVID-19
Hemoglobinopathies such as thalassemia, sickle cell disease as such are not associated with respiratory illnesses in their general symptomatology. However, a major trigger factor precipitating these adverse complications is hypoxia, very commonly seen in COVID-19. Sickle cell disease’s well known complication and major cause of mortality acute chest syndrome is mostly triggered by a respiratory tract infection. Other factors triggering a vaso-occlusive crisis or acute chest syndrome other than hypoxia are acidosis and dehydration [10]. Other complications of Sickle cell disease include chronic haemolytic anaemia, osteopenia, pulmonary hypertension, severe infections, asplenia, kidney failure and others [11].
Patients with sickle cell disease have been hypothesised to be at risk of increased severity of the disease due to the multiple points of overlaps in the pathophysiology with COVID-19, mainly being thrombosis and endovascular inflammation thrombo-inflammation [12].
A positive history of pain is suggestive of a history of episodes of vaso-occlusive crisis prior to the pandemic, making these sickle cell patients, both pediatric as well as adults, at a higher susceptibility especially after a hypoxic triggering infection like COVID-19. Figure 1 shows risk factors in adult and pediatric sickle cell disease patients. Other risk factors could be renal comorbidities, chronic illnesses of heart and lungs, and female sex [13].
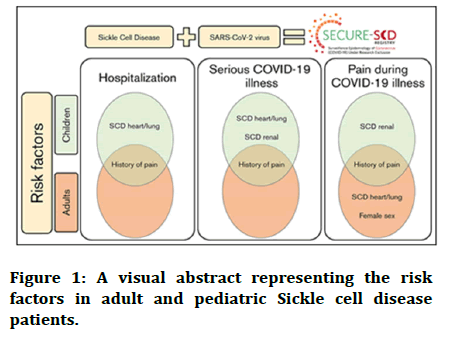
Figure 1: A visual abstract representing the risk factors in adult and pediatric Sickle cell disease patients.
Effect of COVID-19 on blood picture of SCD patients
Based on a study conducted recently on blood indices of COVID-19 patients, most of these patients had a normal blood count and lactate dehydrogenase levels on admission. However, a collective analysis on other available and reliable sources, it has been stated that hemoglobin values substantially reduce in severe cases [14].
Contrary to other viral fevers, like dengue, none of these patients showed a blood picture of thrombocytopenia [15].
Proteomic analysis conducted on SARS-CoV-2 proteins, revealed that these proteins might be binding to porphyrins, formed as a result of dissociation of the heme part of haemoglobin due to attack on its 1-beta chain by the other 3 viral proteins. This ultimately results in formation of deoxyheoglobin, an easier and more susceptible target for viral pathogens, such as SARS-CoV-2, and could also be contributing to the distress symptoms experienced during COVID-19 infection [16].
COVID-19 and sickle cell disease (adults)
A study based on data collected from Brook dale university health system between the dates 18 March to 30 April 2020, on diagnosed patients of age 18 and above with a history of sickle cell disease homozygous trait (HbSS) and heterozygous (HbS, HbC) was done. Their clinical symptoms, laboratory finding, and prognosis were taken into account. A total of 725 patients tested positive by reverse transcriptase polymerisation reaction test in oral and nasopharyngeal swabs. Out of these 725 patients, 9 patients were hospitalised. Majority of the patients presented with symptoms like fever (77.8%), muscle ache (66.7%), cough and back pain. Gastrointestinal symptoms like diarrhoea, dyspnoea and sore throat were seen in a lesser fraction (Figure 2) [17].
Figure 2: Chest radiograph of adult Sickle cell disease patients with positive COVID status.
COVID-19 and pediatric sickle cell disease patients
SCD has shown varied clinical experiences in pediatric age groups. Splenic sequestration, a well-known life threatening complication, also known as ‘splenic-crisis’, has an estimated prevalence of about 12%. The median age of presentation is 1.4 years and mostly seen in the youngest pediatric patient. This factor has been known to be a cause of increased susceptibility to infections [18].
Sickle cell disease patients are known to have an increased susceptibility to infections; however, COVID-19 infection’s effects are not yet very well documented for the pediatric age group. There have also been limited descriptions on acute chest syndrome in the pediatric age group. Documentations on other complications set off in COVID-19 by Sickle cell disease in the pediatric age group are also very minimal [19].
Most common clinical manifestations in children tested positive for COVID-19 were fever and cough. They may be accompanied by other symptoms like general fatigue, upper respiratory tract infections. Headache, dizziness and gastrointestinal symptoms [20].
Comparison of symptomatology in adults and paediatric age groups. A recent study showed that the most pediatric patients presented with chief complaints of pain (35.4%, 120 out of 339 patients), followed by pneumonia (15.3%, 52 out of 339). Pain was also the most common presentation in adult patient group (66.6%, 249 out of 374). Other additional symptoms that were reported were fever, cough, chills, and shortness of breath, myalgia and back pain [21].
Complications seen in sickle cell disease due to COVID-19
Both sickle cell disease and COVID-19 have been known to be associated with clumping of red blood cells that is coagulopathy. These patients have also shown findings similar to those with infection induced inflammatory changes seen in patients of disseminated intravascular coagulopathy. These conditions together pose an increased risk of life threatening condition–Pulmonary Embolism (PE). Computed Tomography Pulmonary Angiogram (CTPA) has been known to be the investigation of choice in patients with symptoms and signs coherent with pulmonary embolism. Based on a retrospective cohort, 23% of patients have been known to have suffered from a pulmonary embolism during their COVID-19 positive status [22].
Some other complications include of COVID-19 and SCD are functional asplenia, posing a higher risk for encapsulated bacterial infections, Iron overload, favouring oxidative stress and causing chronic lung damage [23].
Acute chest syndrome is a pulmonary condition which presents clinically as fever, chest pain, cough and dyspnoea, which can often be misdiagnosed as pneumonia. Delay in the diagnosis can result into possible fatality and hence a highly sensitive tool like HRCT instead of chest X-Ray, where signs can easily be missed, should be done in patients of ACS as well as SCD. Despite COVID-19 being reported to have cause a milder infection paediatric age group, radiological findings of those with a sickle cell genotype seem to resemble those of in adults [24].
Pulmonary function changes in sickle cell disease: Pulmonary functions have been observed to decrease in sickle cell disease patients. Based on older studies conducted on patients of Sickle Cell Trait (SCT), lung functions were compromised as well. The decline in pulmonary function tests has been attributed to the recurrent lung and chest infection leading to alteration in lung parenchyma structure and elasticity of elastic fibres and collagen fibres. The functional parameters affected are FCV (Forced Vital Capacity), FEV (Forced Expiratory Volume), and FEC (Forced Expiratory Capacity). Additional factors could be that pulmonary vasculature is extremely sensitive to micro occlusion driven by hypoxia (a trigger factor commonly seen in COVID-19). Pulmonary micro-occlusion along with cell adhesion can lead to pulmonary hypertension affecting the lung functions further [25].
Sickle cell disease patients are at an increased susceptibility to chest infections due to leucocyte dysfunction and loss in humoral and cell mediated immunity. The major cause of mortality in SCD patients are acute chest syndrome, pneumonia and Acute Respiratory Distress Syndrome (ARDS) [26].
Imaging and presentation of sickle cell disease the following images correspond to a patient study, a 24 years old male with a positive history if minor pain episodes, presenting with chief complaints of severe thoracic pain since 3 days. On examination, the patient was afebrile (37.6°C), pulse rate of 76/min, blood pressure 106/65 mm hg, respiratory rate of 18/min and oxygen saturation of 97% on room air. The patient’s throat and nasopharyngeal swabs tested negative for SARS-CoV-2 and the NCCT was not characteristic of COVID-19 picture due to lack of characteristic ground glass opacities and crazy paving patterns. He was therefore treated for a VOC (complicated with acute chest syndrome) with high flow oxygen, morphine with pain controlled anaesthesia and was discharged on improvement of symptoms.
After 1 day, the patient reported with worsened symptoms including increased chest pain, dyspnoea. The patient on examination was febrile, showed a saturation of 93% on room air and had a respiratory rate of 20/min. the patient’s chest CT also revealed bilateral infiltrates in lower lobes. This time sputum samples were taken, which tested positive for COVID-19. The patient was treated with high flow oxygen, morphine and amoxicillin/clavulanic acid. The patient had a smooth recovery and was discharged after 3 days with instructions of home isolation (Figure 3) [27].
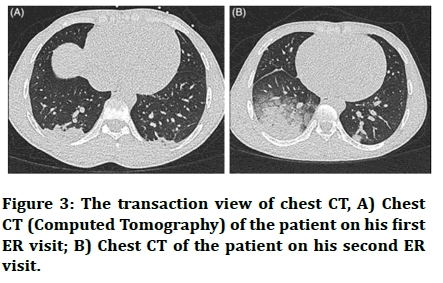
Figure 3: The transaction view of chest CT, A) Chest CT (Computed Tomography) of the patient on his first ER visit; B) Chest CT of the patient on his second ER visit.
Monitoring and treatment of these complications
Sickle cell disease should be considered as a high priority group for COVID-19 testing, as a vulnerable group, an RTPCR as well as CTPA and HRCT should be done as a routine test for these patients showing any symptom of COVID-19. Coagulation profiling of patients, whether newly confirmed or presumptive, should be done, including D-dimer, PT, APTT, platelet count and fibrinogen to provide information for prognosis [28].
Hydroxyuracil (HU) is an FDA approved pharmacological treatment modality for sickle cell disease. Documented reports from published papers have shown that 50% of children and 41.8% adults were on hydroxyuracil therapy when tested positive for COVID-19. The drug has proven to decrease the frequency and intensity of episodes of pain. It has also shown to decrease the occurrence of acute chest syndrome episodes, requirement for transfusion and hospital stay. He drug has proven to be safe, cost effective as well as effective for both genotypes of sickle cell disease [29,30].
HU therapy increases the Fetal Haemoglobin (HbF) levels, decreases HBS levels and reduces the frequency as well as activation of monocytes, thereby decreasing the classic inflammatory response. Since, COVID-19 is known to be associated with cytokine storm and inflammatory lung injury, HU decreases the incidence by decreasing the absolute monocyte count and inflammatory cytokines [30].
A prophylactic pharmacological intervention like low molecular weight heparin is known to have reduced chest pain and delay ACS [31].
High D-dimer levels are being used as an important marker for coagulopathy. High mortality of COVID-19 has been shown to be associated with coagulopathy and thrombosis. Unfractionated heparin and LMWH bind to coronavirus with high affinity and impair its function. Due to this therapeutic nature of LMWH against COVID-19 infection and high plasma protein binding, heparin can become unviable for the desired antithrombotic effect. To prevent this, a sulphated non anticoagulant LMWH with low plasma binding capacity and no bleeding side effects should be considered as an adjunct. Qari, et al. conducted a randomised trial of 253 patients of VOC who either received therapeutic tinzaparin at dose of 175 IV/kg or placebo [32]. Tinzaparin group reported with lesser number of days with severest pain score, days of hospitalisation and overall duration of pain complaints [33].
Other treatment modalities, other than low molecular weight heparin are intravenous fluids, Cefotaxime and Azithromycin, Enoxaparin and antiviral drugs based on the clinical and investigative picture of the patient [34].
Drug toxicity should also be kept in mind while treating these patients. Desferoxamine is also known to be used for Iron chelation. Supportive ventilation using noninvasive ventilation and supplementary oxygen is used for hypoxemic patients to achieve a target peripheral capillary oxygen saturation also known as SpO2 >95% [35].
A newly approved drug for treatment and prevention of vasoocclusive crisis in sickle cell disease patients, drizanlizumab, a monoclonal antibody that binds to the P-selectin proteins, an adhesive molecule that is found on platelets and active endovascular cells. Crizanlizumab binds to this P-selection protein, preventing its interaction with its specific glycoprotein ligand. A randomized blind study, the SUSTAIN trial has shown that Crizanlizumab has decreased the risk of VOC by almost 45% irrespective of the use of hydroxyl uracil as an adjunct. However, its efficacy and safety to be used in pediatric population is yet to be studied [36].
Red Blood Cell (RBC) transfusion or automated exchange transfusion is to be done based on clinical evaluation done by treating physician. The various complications associated with blood transfusions are alloimmunisation, iron overload and increased risk of infection. One of the major known risks in acutely ill patients is VTE (Venous Thromboembolism), risks being 4 times more in sickle cell disease patients [37].
Surgical interventions like splenectomy in general don’t seem to pose a risk for viral infections but will increase the risk of bacterial infections [38].
Optimizing management of sickle cell disease patients during COVID-19 pandemic
Telemedicine has played a trivial role in avoiding unnecessary exposure to SARS-CoV-2. Health care workers should educate their patients on basic preventive methods for COVID-19 like practicing social isolation, given their extreme susceptibility to complications of the disease, hand hygiene and avoiding social gatherings. A few patients with an uncomplicated pain crisis can be managed at home with prescription of oral opioid regimens under close supervision and monitoring of their respective health care providers. Patients should also be motivated to be adherent to disease modifying therapies like hydroxyurea, which has proven to cause a decline in the frequency and occurrence of vaso-occlusive crisis needing medical attention and hospitalization. Routine visits to the hospital should also be avoided. A triage system on phone calls and teleconsultations based on presenting acute complaints should be made. Based on these symptoms, we can schedule visits for the patients requiring medical assistance.
Conclusion
COVID-19 and sickle cell disease show an overlap in their pathophysiology and since, sickle cell disease patients are immunocompromised and have various comorbidities due to their hyper coagulable state, and they are at a relatively higher risk of falling into complications. The overlapping symptoms chest pain or discomfort, dyspnoea and desaturation, can result into error in clinical judgment. When recognised correctly and given adequate supportive care, the disease has shown a positive outcome. Therefore, patients complaining of chest pain should be tested for COVID-19 irrespective of other symptoms like fever, breathlessness, chills, myalgia and cough.
As per the studies conducted, hemoglobin genotype does not seem to be associated with the COVID-19 disease severity. The various hypothesis related to the mild or moderate COVID-19 infection in SCD patients are
• Low humoral immune response due to decreased circulation of CD4+ and CD8+ T lymphocytes. This results in reduced cytokine storm, thus causing a mild severity of the disease.
• Benefit of splenectomy, seen in animal models, due to decrease pro-inflammatory/inflammatory ratio.
• A high ferritin ratio is a negative prognostic factor, since patients with sickle cell disease are on iron chelation therapy due to iron overload, patients on deferipone or desferoxamine might be benefitting from it.
As per literature, comparing overall outcomes of patients of SCD with COVID-19 infection, it was more favourable in children. This is because, micro vascular thrombosis and its related complications, although observed in adults, there have no such evidence in the pediatric population. Therefore, the use of anticoagulants for all patients is still controversial.
Therefore, the overall reported and documented cases of SCD patients with positive COVID-19 infection, has shown to have a relatively positive outcome when diagnosed and given adequate hospital care.
References
- Kehinde TA, Osundiji MA. Sickle cell trait and the potential risk of severe coronavirus disease 2019—A mini‐review. Eur J Haematol 2020; 105:519-523.
- Ashorobi D, Ramsey A, Yarrarapu SNS, et al. Sickle Cell Trait. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2019.
- Panepinto JA, Brandow A, Mucalo L, et al. Coronavirus disease among persons with sickle cell disease, United States, March 20–May 21, 2020. Emerg Infect Dis 2020; 26:2473.
- Sayad B, Karimi M, Rahimi Z. Sickle cell disease and COVID‐19: Susceptibility and severity. Pediatr Blood Cancer 2021; 68:29075.
- Gupta A, Chaudhary K, Kaushik R. Role of Emergency Automated Red Cell Exchange in Sickle Cell Crisis: A Case Report. Clin Med Insights Case Rep 2020; 13.
- Heilbronner C, Berteloot L, Tremolieres P, et al. Patients with sickle cell disease and suspected COVID‐19 in a pediatric ICU. Br J Haematol 2020; 190: e21-e24.
- Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020; 135:2033-2040.
- Kehinde TA, Osundiji MA. Sickle cell trait and the potential risk of severe coronavirus disease 2019—A mini‐review. Eur J Haematol 2020; 105:519-523.
- Sivalingam T, Inusa B, Doyle P, et al. COVID‐19 and the pulmonary complications of sickle cell disease. EJHaem 2020; 1:545-547.
- Corrons JL, De Sanctis V. Rare anaemias, sickle-cell disease and COVID-19. Acta Biomed 2020; 91:216.
- Ramachandran P, Perisetti A, Kathirvelu B, et al. Low morbidity and mortality with COVID‐19 in sickle cell disease: A single centre experience. EJHaem 2020; 1:608-614.
- Chowdhury SF, Anwar S. Management of hemoglobin disorders during the COVID-19 pandemic. Front Med 2020; 7:306.
- Mucalo L, Brandow AM, Dasgupta M, et al. Comorbidities are risk factors for hospitalization and serious COVID-19 illness in children and adults with sickle cell disease. Blood Adv 2021; 5:2717-2724.
- Van Tuijn CF, Nur E, van Beers EJ, et al. Acute chest syndrome in sickle cell disease due to the new influenza A (H1N1) virus infection. Am J Hematol 2010; 85:303-304.
- Hussain FA, Njoku FU, Saraf SL, et al. COVID‐19 infection in patients with sickle cell disease. Br J Haematoly 2020; 189:851-852.
- Davies SC, Win AA, Luce PJ, et al. Acute chest syndrome in sickle-cell disease. Lancet 1984; 323:36-38.
- Chowdhury SF, Anwar S. Management of hemoglobin disorders during the COVID-19 pandemic. Front Med (Lausanne) 2020; 7:306.
- Nur E, Gaartman AE, van Tuijn CF, et al. Vaso‐occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID‐19). Am J Hematol 2020; 95:725.
- Appiah‐Kubi A, Acharya S, Levy CF, et al. Varying presentations and favourable outcomes of COVID‐19 infection in children and young adults with sickle cell disease: an additional case series with comparisons to published cases. Br J Haematol 2020.
- Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost 2020; 18:1559-1561.
- Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med 2000; 342:1855-1865.
- Godeau B, Schaeffer A, Bachir D, et al. Bronchoalveolar lavage in adult sickle cell patients with acute chest syndrome: value for diagnostic assessment of fat embolism. Am J Respir Crit Care Med 1996; 153:1691-1696.
- Vichinsky EP, Styles LA, Colangelo LH, et al. Cooperative Study of Sickle Cell Disease. Acute chest syndrome in sickle cell disease: clinical presentation and course. Blood 1997; 89:1787-1792.
- Varacallo M, Mair SD. Superior Labrum Anterior Posterior Lesions. StatPearls Publishing, Treasure Island (FL). 2020.
- John N, Jyoti J, Niraimathi D, et al. Lung functions and oxidative status in sickle cell disease and sickle cell trait. Int J Clin Exp Physiol 2014; 1:273.
- John NA, John JE. Implications of COVID-19 infections in sickle cell disease. Pan Afr Med J 2020; 36.
- Nur E, Gaartman AE, van Tuijn CF, et al. Vaso‐occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID‐19). Am J Hematol 2020; 95:725.
- Sahu KK, Siddiqui AD, Cerny J. Managing sickle cell patients with COVID-19 infection: the need to pool our collective experience. Br J Haematol 2020; 190: 86-e89.
- Elia GM, Angel A, Regacini R, et al. Acute chest syndrome and COVID-19 in sickle cell disease pediatric patients. Hematol Transfus Cell Ther 2021; 43:104-108.
- Alsayegh F, Mousa SA. Challenges in the management of sickle cell disease during SARS-CoV-2 pandemic. Clin Appl Thromb Hemost 2020; 26:1076029620955240.
- Chakravorty S, Padmore-Payne G, Ike F, et al. COVID-19 in patients with sickle cell disease–a case series from a UK tertiary hospital. Haematol 2020; 105:2691.
- Vilela TD, Braga JA, Loggetto SR. Hemoglobinopathy and pediatrics in the time of COVID-19. Hematol Transfus Cell Ther 2021; 43:87-100.
- Esposito S, Marchetti F, Lanari M, et al. COVID-19 management in the pediatric age: Consensus document of the covid-19 working group in paediatrics of the Emilia-Romagna Region (re-co-ped), Italy. Int J Environ Res Public Health 2021; 18:3919.
- Jacob S, Dworkin A, Romanos‐Sirakis E. A pediatric patient with sickle cell disease presenting with severe anemia and splenic sequestration in the setting of COVID‐19. Pediatr Blood Cancer 2020.
- Corrons JL, De Sanctis V. Rare anaemias, sickle-cell disease and COVID-19. Acta Biomed 2020; 91:216.
- Ramachandran P, Perisetti A, Kathirvelu B, et al. Low morbidity and mortality with COVID‐19 in sickle cell disease: A single center experience. EJ Haem 2020; 1:608-614.
- Menapace LA, Thein SL. COVID-19 and sickle cell disease. Haematol 2020; 105:2501.
- Corrons JL, De Sanctis V. Rare anaemias, sickle-cell disease and COVID-19. Acta Biomed 2020; 91:216.
Author Info
Tanvi Punj and Sarika Dakhode*
Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (Demmed to be University) Sawangi (Meghe) Wardha, Maharashtra, IndiaCitation: Tanvi Punj, Sarika Dakhode, Consequences of COVID-19 in Sickle Cell Disease Patients, J Res Med Dent Sci, 2022, 10 (9): 198-203.
Received: 01-Jul-2022, Manuscript No. JRMDS-22-49567; , Pre QC No. JRMDS-22-49567(PQ); Editor assigned: 04-Jul-2022, Pre QC No. JRMDS-22-49567(PQ); Reviewed: 18-Jul-2022, QC No. JRMDS-22-49567; Revised: 01-Sep-2022, Manuscript No. JRMDS-22-49567(R); Published: 08-Sep-2022
