Research - (2021) Volume 9, Issue 8
COVID 19 Presentation and Effect of Associated Co-morbidities on Severity of Illness at a Dedicated COVID Hospital in North India
Devendra Kumar Singh1, Ankur Garg1, Sharad Bagri1, Prashant Choudhary1*, Dheerendra Kuber2 and Anil Kumar Kem3
*Correspondence: Prashant Choudhary, Department of Respiratory Medicine, School of Medical Sciences & Research, India, Email:
Abstract
Background: Corona virus (SARS-Cov-2) belongs to coronaviridiae family, first identified in December 2019 in Wuhan, China, thus named as corona virus disease-19 (COVID-19). Later on the World Health Organization declared the outbreak as Public Health Emergency of International Concern on 30 January 2020 and the pandemic on 11 March. Material and methods: A total of 332 patients, diagnosed with COVID-19 by Reverse transcription polymerase chain reaction ( RT-PCR), admitted in level iii, dedicated COVID hospital were evaluated for presenting symptoms and various comorbidities by detail history, body mass index, random blood sugar, x-ray chest, electrocardiography and kidney function test. Severity of disease was stratified as mild, severe, critical and mortality. Results: Of 332 patients, 205 (61%) were males and 127(38.3%) were females with average age 40.21 ± 16.15. 298 (89.8%) from urban area and 34 (10.2%) from rural area. Most common symptom was fever, in 192 (57.8%) patients, followed by cough, 136 (41.0%), sore throat, 95 (28.6%). The least common symptoms were rhinorrhoea (11%) and vomiting (11%). As per severity of disease, 251 (75.6%) were mild, 45 (13.6%) severe, 11 (3.3%) critical, and 12 (3.6%) expired. Conclusion: Older cases were at a risk of developing severe and critical illness. Males were found to have a more severe illness. Smokers have severe illness, compared to non-smokers, and the association between severity and smoking was significant. There was a fair and significant correlation between number of comorbidities and severity of illness. Among the comorbidities, chronic kidney disease has strongest association with mortality.
Keywords
COVID 19, pandemic, prevalence, RT-PCR, comorbiditiesIntroduction
Coronavirus (CoV) is clustered under the viral family group that causes disease in mammals and birds. A pandemic novel coronavirus was named as ‘‘Corona Virus Disease 2019’’ (2019-nCoV) by World Health Organization (WHO) in Geneva, Switzerland [1-3]. As its RNA pattern is closer to SARS, the 2019 Coronavirus is renamed as SARSCoV- 2 pandemic. It belong to the subfamily Orthocoronavirinae inside the family Coronaviridae, order Nidovirales, and the realm Riboviria [4]. A twodimensional view of Corona beneath a transmission electron microscopy reveals a characteristic look of ‘‘paying homage to a crown’’ around the virions [2]. This lead to naming the virus ‘‘Corona’’, meaning ‘‘crown’’ or ‘‘halo’’ in Latin
This is the deadly third-generation virus in Corona family preceded by Severe Acute Respiratory Syndrome (SARS) in 2003, killed almost 10% of total affected patients (8429) across 29 international locations and Middle East Respiratory Syndrome (MERS) in 2012, even more lethal with a mortality rate of 30% of the infected patients [5].
In 1937, the primary coronavirus was located in bats, rarely affecting humans and mostly circulating among animals like bats, camels, and cats. Later, they mutated to contaminate rats, cattle, pigs, mice, cats, dogs, horses, and turkeys. The first human coronavirus, known in the sixties, infected the nasal cavities of humans resulting in common cough and cold [6].
The COVID-19 outbreak was first identified in December 2019 in Wuhan, China [7,8]. The World Health Organization declared the outbreak a Public Health Emergency of International Concern on 30 January 2020 and a pandemic on 11 March [9,10]. The first case of COVID-19 in India, which originated from China, was reported on 30 January 2020. India currently has the largest number of confirmed cases in Asia [11], and has the third highest number of confirmed cases in the world after the United States and Brazil [12].
Presenting signs and symptoms of COVID-19 vary. Most persons experience fever (83–99%), cough (59–82%), fatigue (44–70%), anorexia (40–84%), shortness of breath (31–40%), myalgia (11–35%). Other non-specific symptoms, such as sore throat, nasal congestion, headache, diarrhoea, nausea and vomiting, have also been reported [8,13]. Loss of smell (anosmia) or loss of taste (ageusia) preceding the onset of respiratory symptoms has also been reported [14,15]. The association of these symptoms with disease is not clear. The association of any specific symptom or comorbidity with severity of disease and mortality is still not clear.
In our study we have done research on COVID 19 presentation and effect of associated comorbidities on severity of illness at a dedicated, level 3 COVID hospital in North India. We have done a detailed analysis of 332 COVID positive patients admitted to our hospital, regarding their signs, symptoms and comorbidities .This study will result in a better understanding of this life threatening virus as no such study has been conducted before in a tertiary care hospital in our region, thereby helping us to find better precaution and treatment.
Materials and Methods
Study design
This is a descriptive, cross sectional study on admitted patients of COVID 19 Study period: The study was carried out from 22nd May 2020 to 21st July 2020.
Setting
Present study was conducted at a level 3 COVID 19 centre in north India.
Study population
This study was conducted on 332 patients of SARS Cov 2, diagnosed by Reverse transcription polymerase chain reaction ( RT-PCR), admitted at a level 3, dedicated COVID centre in North India.
Sampling
All patients, admitted during study period were enrolled into the study.
Sampling size
According to study ‘Clinical characteristics of coronavirus disease 2019 in China, by Guan et al. [16].
It was observed that the prevalence of fever was 88% amongst diagnosed cases of corona virus disease (COVID 19 disease).
Assuming value of α to be 0.05 and allowable error in the estimate of 5%, sample size was calculated using formula: n=Z2pq/ d2, Z=1.96, p=prevalence (88% or 0.88), q=1-p (12% or 0.12), d=permissible error (5% or 0.05), n=sample size, Thus, sample size came out to be 162.26. 10% for each variable was added, which leads to the sample size 324.52. Finally we enrolled 332 patients in this study.
Inclusion criteria
All admitted patients, who were diagnosed with COVID 19, irrespective of gender, religion, smoking status, residence, socioeconomic status, educational qualification and comorbidities, willing to participate in this study, having no exclusion criteria were included in this study.
Exclusion criteria
Patients of age less than 10 years or not willing to participate in our study were excluded from this study.
Data variables
All cases of COVID 19, diagnosed on the basis of RT-PCR of oropharyngeal or nasopharyngeal swab, admitted in a dedicated COVID hospital were evaluated for their demographic features (Age, Gender and Urban or Rural residence) symptoms (Fever, Chills/Rigor, Cough, Fatigue, Anorexia, Myalgia, Sore throat, Rhinorrhoea, Nausea, Vomiting, Diarrhoea, Headache, Dyspnoea, Sputum production, Anosmia and Ageusia).
All cases were also evaluated for comorbidities and other conditions {cardiovascular disease (CAD), Diabetes mellitus (DM), Hypertension (HTN), chronic lung disease, Chronic kidney disease (CKD), Cancer, Obesity and Smoking}
Spectrum of disease was stratified as Mild – Low grade fever, cough, malaise, rhinorrhoea, sore throat, shortness of breath, haemoptysis, GI symptoms like nausea, vomiting, diarrhoea, Without any change in mental status, and non immunocompromised. Severe – Respiratory rate >30/min, SpO2 <93%, PaO2/FiO2 <300, Lung infiltrates >50% within 24-48 hours. Critical – Respiratory failure (need of mechanical ventilation), septic shock, MODS. Mortality – Any death of COVID 19 diagnosed case irrespective of co morbidities [17].
Data collection tools
Face to face or telephonic interview using open and semi open questions to evaluate different aspects of history and symptoms were used. Lab investigations such as Xray chest, CBC, RFT, ECG, Blood sugar and BMI calculation of all patients were done.
Statistical analysis
Data was collected, entered and cleaned in MS Excel. Data was analysed using SPSS (Statistical Programme for Social Sciences) Version 16. Categorical data was presented in the form of percentages and continuous data was presented in the form of means and standard deviation. Chi-square test was used to assess significance in outcomes of categorical data, while significance in continuous data was assessed using Independent Samples t-test. Correlation scores were analysed using Spearman Rank Correlation Test.
Calibration of examiners was done to reduce intra examiner variability. Coordination among all investigators was maintained during study period to maintain the quality of data. Validation of data was done by random selection of 5% data and revaluation.
Approval was taken from faculty research board and institutional ethical committee. Nature of study was explained to all participants and written consent was taken.
Results
A total of 332 patients of SARS COV 2 were evaluated. The mean age of the study participants in our study was 40.21 years. There was a preponderance of male patients in our study, with more than three-fifths of the recruited cases being men. The average body mass index was, mean (SD) 24.57 (3.00), median 25 and range 13-36. Majority of them, 298 (89.8%) were from urban area while only 34 (10.2%) were from rural area. 276 (63.0%) were non-smoker and 56 (16.9%) were smoker. As figure1 shows, majority of cases (64.4%) were in age group 20-29 years while very few patients in age group above 70 years, only 6%.
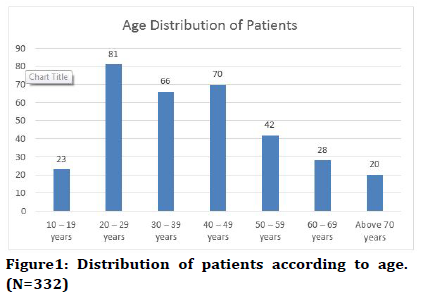
Figure 1. Distribution of patients according to age. (N=332)
As Table1 shows, most common symptom in our study was fever, in 192 (57.8%) patients, followed by cough, in 136 (41.0%) patients and sore throat, in 95(28.6%), while least common symptoms were rhinorrhoea and vomiting, only 11% each.
| Symptoms | Number of cases presenting (%) |
|---|---|
| Fever | 192 (57.8) |
| Chills/Rigor | 18 (5.4) |
| Cough | 136 (41.0) |
| Fatigue | 60 (18.1) |
| Anorexia | 15 (4.5) |
| Myalgia | 27 (8.1) |
| Sore Throat | 95 (28.6) |
| Rhinorrhoea | 11 (3.3) |
| Nausea | 16 (4.8) |
| Vomiting | 11 (3.3) |
| Diarrhoea | 23 (6.9) |
| Headache | 80 (24.1) |
| Shortness of Breath | 74 (22.3) |
| Sputum in cough | 23 (6.9) |
| Anosmia | 45 (13.6) |
| Ageusia | 39 (11.7) |
Table 1: Symptoms as reported at the time of admission and during hospitalization (N=332).
Majority of the cases in our study did not have even a single co-morbidity; however, one-third of the cases suffered from at least one chronic illness. Almost onefifth of the participants had a history of hypertension, while almost 17% cases had a history of diabetes mellitus. 9% cases also had a history of a cardiovascular event. Twenty four cases had a history of chronic kidney disease (Figure 2).
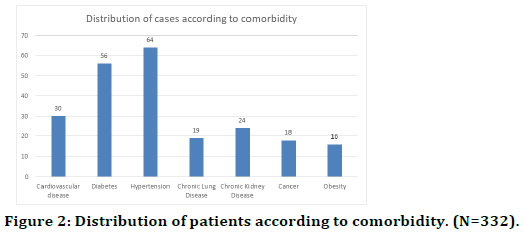
Figure 2. Distribution of patients according to comorbidity. (N=332).
As Table 2 shows, three-fourth of the COVID-positive cases recruited in our study had a mild course of disease, 45 (13.6%) had severe disease and only 11 (3.3%) patients were having critical disease. 12 (3.6%) patients recruited in this study died.
| Severity of Disease | Number of cases reporting (%) |
|---|---|
| Mild | 251 (75.6) |
| Severe | 45 (13.6) |
| Critical | 11 (3.3) |
| Mortality | 12 (3.6) |
Table 2: Distribution of patients according to severity of the disease (N=332).
We analysed the association of mortality with socioclinical characteristics of the patients. There was no significant association of age, residential status or body mass index of the patient with mortality. Comparable proportion of men and women died in our study group. Amongst symptoms that showed significance with mortality, an increased proportion of patients who reported anorexia, nausea and vomiting died compared to patients who did not have these symptoms. Similar association was seen for patients who reported having shortness of breath (Table 3).
| Symptoms in patients who died (N=12) | Had Symptom (%) | Did not have symptom (%) | p-value |
|---|---|---|---|
| Fever | 7 (3.9%) | 5 (3.6%) | 0.971 |
| Chills/Rigor | 0 | 12 (4.0%) | 0.398 |
| Cough | 8 (6.5%) | 4 (2.1%) | 0.065 |
| Fatigue | 1 (1.7%) | 11 (4.3%) | 0.372 |
| Anorexia | 2 (13.3%) | 10 (3.3%) | 0.04 |
| Myalgia | 0 | 12 (4.1%) | 0.294 |
| Sore Throat | 3 (3.2%) | 9 (4.0%) | 0.773 |
| Rhinorrhoea | 1 (9.1%) | 11 (3.6%) | 0.322 |
| Nausea | 3 (18.8%) | 9 (3.0%) | 0.001 |
| Vomiting | 2 (16.7%) | 10 (3.3%) | 0.008 |
| Diarrhoea | 2 (8.7%) | 10 (3.4%) | 0.176 |
| Headache | 11 (4.6%) | 1 (1.3%) | 0.193 |
| Shortness of Breath | 9 (13.0%) | 3 (1.2%) | 0.001 |
| Sputum in cough | 1 (4.3%) | 11 (3.7%) | 0.848 |
| Anosmia | 1 (4.3%) | 11 (3.7%) | 0.59 |
| Ageusia | 1 (2.6%) | 10 (3.6%) | 0.778 |
Table 3: Association of symptoms with mortality (N=332).
Association of comorbidities with mortality in SARS CoV-2 was also assessed. Hypertension and chronic kidney disease were found to be significantly associated with mortality due to SARS CoV-2. The average number of comorbidities in patients who died was higher than in patients who did not die, but this association was not significant (Table 4).
| Co-Morbidity | Died (%) (N=12) | Did not die (%) (N=306) | p-value |
|---|---|---|---|
| Cardiovascular disease | 2 (6.7%) | 28 (93.3%) | 0.348 |
| Diabetes | 2 (3.6%) | 50 (96.4%) | 0.985 |
| Hypertension | 5 (7.8%) | 59 (92.2%) | 0.045 |
| Chronic Lung Disease | 1 (5.3%) | 18 (94.7%) | 0.692 |
| Chronic Kidney Disease | 3 (12.5%) | 21 (87.5%) | 0.015 |
| Cancer | 1 (5.6%) | 17 (94.4%) | 0.65 |
| Obesity | 1 (6.2%) | 15 (93.8%) | 0.563 |
Table 4: Association of mortality with co-morbidity (N=332).
Association of severity of illness with socio-clinical characteristics of patients was also analysed. Age was significantly correlated with severity of illness, indicating older cases were at a risk of developing severe and critical illness. Independently, we found BMI to be significantly correlated with Age. Hence, correlation of BMI was also found to be significant with severity of illness (Table 5).
| Correlation Score | p-value | |
| Age of Patients | 0.259 | <0.001 |
| BMI | 0.096 | 0.082 |
| Sex | -0.053 | 0.337 |
| Rural/Urban | -0.028 | 0.616 |
| Smoker/Non-Smoker | 0.147 | 0.007 |
| Number of comorbidities | 0.301 | <0.001 |
Table 5: Correlation of patient characteristics with severity of disease (N=332).
Discussion
The current set of clinical and epidemiological characteristics of 332 patients have exhibited varied pattern in the tropical area of North India that could help in better management of COVID-19 patients and may give a platform to evolve better public health strategies to flatten the uprising upstroke of the epidemic curve and decelerate the explosive splurge and surge of COVID-19 disease.
It could be perceived that patients in the age range of 20–49 years, comprising 217 (65.3%) of the sample patients, were maximally afflicted. Since, the risk of comorbidities increases with increasing age, thus, comorbidities can be considered as a confounding factor between age and severity of illness
Since, no home quarantine was allowed in the state of Uttar Pradesh during the study period, thus most of the patients were admitted either to quarantine centre or to the COVID dedicated hospitals.
Thus, almost all the patients, admitted to Sharda hospital (a level iii, dedicated COVID Hospital) were symptomatic. The percent of patients who presented with fever was 57.8%, cough was 41%, and 28.6% of patients had complaints of sore throat. Cia et al. [18], though included only ten patients in their study, too had observed that most common symptom occurred during the early days of outbreak in paediatric population at Wuhan was fever 80% (eight in number), followed by cough 60% (six in number), sore throat 40% (four in number), stuffy nose 30% (three in number), sneezing and rhinorrhea 30% (three in number).
A similar sign and symptom complex across geographical locales of temperate region of Wuhan, China, and tropical area of India upholds further the premise that such an array of sign and symptoms could act as a first public screening measure and tool to assay suspects with SARS-CoV-2 infection.
Though, majority of patients in the present study didn’t suffer from any of the co-morbidity. But the most common co-morbidity was Hypertension (19.2%), followed by diabetes (15.6%). A kumar et al. [19] in their meta-analysis concluded that the diabetes is associated with a two-fold increase in mortality as well as severity of COVID-19, as compared to non-diabetics.
In the present study, it was observed that the most common co-morbidities that had significant association with mortality were Hypertension and Chronic kidney disease.
Diabetes, though, being the second most common comorbidity was not significantly associated with mortality.
From this study, it can be concluded that the COVID-19 disease has low mortality rate in our county as compared to China and other countries.
G Zhang et al [20] in their study on 221 patients concluded that 5.4% of the sample patients expired, whereas in our study, the mortality rate was only 3.6%.
Though, a sufficient number of sample patients were included in the study, significant association between multiple co-morbidities and mortality of COVID-19 patients could not be assessed. More research is required to observe the association between various comorbidities, age, and mortality of patients suffering from COVID-19 disease.
Conclusion
The negative correlation of sex with severity of disease indicates males were predisposed to a more severe illness. However, the correlation was not significant. Similarly, people living in rural areas were also likely to develop severe illness; however the relationship between either of the variables was not significant. Smokers were predisposed to developing a severe illness, compared to non-smokers, and the association between severity and smoking was significant. There was a fair and significant correlation between number of comorbidities and severity of illness.
Fever, cough and shortness of breath were significantly correlated with severity of illness. Gastro-intestinal symptoms like nausea, vomiting, diarrhoea, and anorexia had a fair and significantly positive correlation with severity of illness, indicating GI symptoms to be a better correlate of severity than respiratory symptoms. Cardiovascular diseases and hypertension were also significantly correlated with severity of illness.
References
- Log S. Understanding SARS-CoV-2. 2020; 1-9.
- Zhang C, Zheng W, Huang X, et al. Protein structure and sequence re-analysis of 2019-nCoV genome does not indicate snakes as its intermediate host or the unique similarity between its spike protein insertions and HIV-1. J Proteome Res 2020; 19:1351-160.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270-3.
- Maier HJ, Walker JM. Coronaviruses IN Series Editor.
- https://asm.org/Articles/2020/January/2019-Novel-Coronavirus-2019-nCoV-Update-Uncoating
- Zhang L, Liu Y. Potential interventions for novel coronavirus in china: A systematic review. New York: Wiley; 2020.
- https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497-506.
- https://www.who.int/news/item/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020
- https://www.hindustantimes.com/india-news/india-most-infected-by-covid-19-among-asian-countries-leaves-turkey-behind/story-Jjd0AqIsuL3yjMWg29uJ3I.html
- https://www.deccanherald.com/national/india-becomes-third-worst-affected-country-by-coronavirus-overtakes-russia-857442.html
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020; 395:507-513.
- Spinato G, Fabbris C, Polesel J, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020.
- Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study. Clin Infect Dis 2020.
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708-1720.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA 2020.
- Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin Infect Dis 2020.
- Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr 2020; 14:535-545.
- Zhang G, Hu C, Luo L, et al. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol 2020; 127:104364.
Author Info
Devendra Kumar Singh1, Ankur Garg1, Sharad Bagri1, Prashant Choudhary1*, Dheerendra Kuber2 and Anil Kumar Kem3
1Department of Respiratory Medicine, School of Medical Sciences & Research, Gautam Buddh Nagar, India2Department of Cardiology, ABVIMS & RML Hospital, New Delhi, India
33Department of Medicine, SIMS, Hapur, India
Citation: Devendra Kumar Singh, Ankur Garg, Sharad Bagri, Prashant Choudhary, Dheerendra Kuber, Anil Kumar Kem,COVID 19Presentation and Effect of Associated Co-morbidities on Severity of Illness at a Dedicated COVID Hospital in North India, J Res Med Dent Sci, 2021, 9(8):49-54
Received: 02-Jul-2021 Accepted: 02-Aug-2021
