Research - (2019) Volume 7, Issue 3
Effect of Sodium Hypochlorite and Sodium Ascorbate on the Shear Bond Strength of Composite to the Dentin of Primary Teeth
Razieh Meshki1, Fateme Zarouni2* and Parisa Sarikhani2
*Correspondence: Fateme Zarouni, Pediatric Dentistry Resident, Department of Pediatric Dentistry, School of Dental Medicine, Ahvaz Jundishapur University of Medical Sciences, Iran, Email:
Abstract
Introduction: Endodontic irrigants affect the dentin properties and bond strength to resin materials. The main purpose of this research was to investigate the effect of sodium hypochlorite and sodium ascorbate on shear bond strength of composite to the dentin of primary teeth.
Materials and Methods: For data collection, the observation method and calculation of shear bond strength between composite and dentin surfaces were used. A total of 40 anterior primary teeth with healthy crown and caries free were studied in 4 groups of 10 as a statistical population.
Results: The shear bond strength of resin to the dentin surface of the teeth was calculated and analyzed. The highest shear bond strength was in normal saline group with a mean as much as 31.95 ± 4.90 and the lowest bond strength was in the sodium hypochlorite group with a mean as much as 12.71 ± 3.04. The use of sodium ascorbate after sodium hypochlorite reversed the compromised bond strength (28.76 ± 5.34). Tukey test was used in two groups to compare the shear bond strengths of composite to dentin.
Conclusion: The results showed that the use of sodium hypochlorite (5.25%) reduced the shear bond strength of composite to dentin and the use of 10% ascorbate sodium could compensate for the reduction of bond strength.
Keywords
Sodium ascorbate, Sodium hypochlorite, Shear strength, Composite, Dentin of primary teeth
Introduction
The aesthetic of the dental system is important for the appearance of a person and gives the person a sense of confidence [1]. Nowadays, composite resins, due to their good appearance and the same color of the tooth and their strong bonding to dental structures are common dental material in restorative dentistry. These materials should be able to resistance the forces applied to the tooth and have sufficient adhesion to dental surface. One of the important factors in creating bonding strength of composite materials is dental structure, which varies in different parts of it. Primary and permanent teeth are different in terms of morphology, enamel and dentin compositions, structures, and components. In primary teeth, dentin is less mineralized and calcium and phosphorus concentrations in tubular and interbutal dentin are less. On the other hand, the number of dentinal tubules and the diameter of these tubules in the primary teeth are less than permanent teeth and have less penetrating potential [2-4]. In the same conditions, the strength and power of different types of dentin bonding to the dentin of the primary teeth are less than permanent teeth [3]. One of the common processes in pediatric dentistry is root canal therapy, and its success strongly relies on mechanical-chemical debridement through endodontic irrigants [5].
The most common endodontic irrigants is sodium hypochlorite (NaOCl), which is used due to its physicochemical properties as an antibacterial and tissuesoluble agent [6]. Sodium hypochlorite is a non-specific proteolytic agent that has the ability to remove organic material, as well as magnesium and carbon ions [7]. It also has unique capacity to dissolve organic components of the smear layer for better penetration of the irrigants and disinfectant fluids into dentinal tubules [8].
Despite the advantages of using sodium hypochlorite, the use of this material may reduce the sealing and adhesion ability of dental materials such as resin base cement and root canal sealer. It has also been shown that the coronal bonding strength of some adhesive materials is reduced by the use of this material [9-11]. The effect of chemical irrigant soluble on dentin is not exactly known, but sodium hypochlorite appears to be broken down when it reacts to sodium chloride and oxygen molecules and the presence of oxygen molecules as a strong inhibitor of polymerization of resin materials. Also, the presence of oxygen cavities in the bonding surfaces of resins to dentin prevents resin penetration into tubular cavities and reduces the bond strength of resin to dentin [12]. The free residual radicals resulting from the use of sodium hypochlorite with free radicals of vinyl chloride compete during the optical activation phase and polymerization is not properly performed [13]. Under such conditions, the best way to eliminate free radicals and oxygen molecules is to use reducing agents, which neutralizes free radicals and oxygen molecules. One of these materials is ascorbic acid and its salts, including sodium ascorbate which has no toxicity and is used in many food products [14]. Regarding the antioxidant activity of this substance, it seems that the use of this material provides bonding strength before bonding procedures [15]. It has also been shown that the use of 10% ascorbate sodium increases the strength of the shear bond of resin to dentin [16].
Regarding the structural difference between permanent and primary teeth and the absence of a study on the effect of sodium ascorbate on shear bond strength of composite to the dentin of primary teeth and the existence of contradictory results on the effect of sodium hypochlorite on bond strength, the purpose of this study was to evaluate the effect of Sodium Ascorbate and Sodium hypochlorite on the shear bond strength of the composite to the dentin of primary teeth.
Materials and Methods
The present study was carried out using experimental methods. The method of observing and calculating the shear bond strength between the composite and dentin surfaces was tested in the universal testing machine to collect data. A total of 40 anterior primary teeth with intact crown with at least 1/3 root canals were collected. The teeth were then kept in disinfectant solution of 0.5% chloramine (sigma alldrich, USA) for one week and the samples were kept in distilled water (Shahid Ghazi, Tabriz-Iran) and distilled water was changed periodically. Labial surfaces of all teeth were removed to expose dentin using high speed bur (Tizkavan, Iran).
Groups
The teeth were randomly divided into four groups:
Group 1: This group was considered as a positive control group and the surface of teeth was washed with normal saline.
Group 2: 5.25% sodium hypochlorite (Poland, cerkamed, chloraxid) was applied for 1 minute on the teeth surface.
Group 3: 10% sodium ascorbate solution (sigma alldrich, USA) for 2 minutes was applied to the teeth surface.
Group 4: After applying 5.25% sodium hypochlorite (Poland, cerkamed, chloraxid) for 1 minute on the teeth surface, 10% ascorbate solution was applied on the teeth surface for 2 minutes.
After application of 37% phosphoric acid gel (vericam, denfill, Korea) for 15 seconds in each group, the dentin surface were washed with water and dried using a cotton pellet to remove excess water. Then, two bonding layers (Single Bond, 3M, ESPE, USA) was dispensed onto a clean disposable brush and the adhesive was applied on the surface of the teeth according to the manufacturer's instructions. Bonding was spread on the dentin surface for 5 seconds with gentle air drying for at least 5 seconds and adhesive was light cured for 20 seconds with LED (Woodpecker, LED, China). Then, composite (Z250, 3M, ESPE, USA) button with an internal diameter of 4 mm and a height of 3 mm placed to the dentin surface and cured for 40 seconds using. The samples were kept in distilled water at room temperature for 24 hours and mounted in acrylic cylinder. Then all specimens tested for the shear bond strength using Instron testing machine and the knife edge shearing chisel was employed to debond the prepared buttons of composite resin force applied perpendicular to the dentin-composite interface at a shear rate of 0.5 mm/min and the numbers related to the shear bond strength of each tooth were calculated [2].
Method for calculating sample size and sampling method
According to Bahrololoomi et al. [17], the mean and standard deviation of shear bond strength in the control group was as much as 38.4 ± 10.5 in the control group and 53.3 ± 9.7 in the experimental group. The sample size was calculated from Equation 1 with a confidence level of 95% and a test power of 90%:
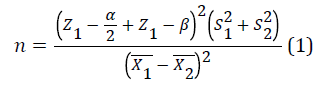
For this purpose, 10 teeth were calculated for each group. Totally, 40 teeth were selected.
How to conduct the research
A total of 40 anterior teeth with intact crown and caries were collected. To prepare samples, the labial surfaces of these teeth were used. The teeth were belonging to a same age group as much as possible. The collected teeth were thoroughly washed in flowing water so that the blood and sticky substances were removed. The teeth were kept disinfected in chlorine t-5% disinfectant solution for one week and then kept in distilled water to prevent dehydration. Water was constantly changed [18]. In order to expose the dentinal surface of the labial, 1mm of enamel was removed by diamond bur with water cooling. In this study, the surface area of dentin was used because the density and diameter of the tubules rise near the pulp and this could affect the ability of the bonding system [19]. The teeth were randomly divided into four groups of 10 teeth.
Each of the groups was prepared before bonding the composite in the following ways:
Group 1: This group was considered as a positive control group and the level of teeth was washed with normal saline.
Group 2: The teeth were placed in a solution of sodium hypochlorite (5.25%) for one minute so that all exposed dentin surfaces are in full contact with sodium hypochlorite. Then the teeth were washed with normal saline and prepared for bonding.
Group 3: In this group, sodium ascorbate powder was measured by a digital scale of the pathology department of the faculty of dentistry (Japan A & D Company) and 10% sodium ascorbate solution was immediately prepared prior to floating teeth inside it using distilled water. The teeth were then placed in a 10% sodium ascorbate solution for 2 minutes. The solution was then removed and then washed with distilled water for bonding procedures.
Group 4: After applying 5.25% sodium hypochlorite for 1 minute on the dentin surface of teeth and rinsing with normal saline, 10% sodium ascorbate solution was placed on the dentin surface for 2 minutes. The solution was then removed and then washed with distilled water for bonding steps.
In each group, the teeth were somewhat dried after rinsing. 37% phosphoric gel (vericam, denfill, Korea) was applied on teeth for 15 seconds. The teeth were washed with water and dried with cotton pellet to remove excess water. Then, two bonding layers (Single Bond, 3M, ESPE, USA) was dispensed onto a clean disposable brush and the adhesive was applied on the surface of the teeth according to the manufacturer's instructions. Bonding was spread on the dentin surface for 5 seconds with gentle air drying for at least 5 seconds and adhesive was light cured for 20 seconds with LED (Woodpecker, LED, China). Composite resin A2 (Z250, 3M, ESPE, USA) was placed in identical transparent cylinders with a diameter of 4 mm and a height of 3mm on the teeth and cured for 40 seconds. Re-curing was performed for 20 seconds to ensure polymerization of the composite after separating the transparent. The samples were kept in distilled water for 24 hours at 37°C.
The samples were mounted in acrylic resin using a cylindrical generator, which are 1mm below CEJ in acrylic. Subsequently, the samples were subjected to 5 and 55 degrees of 1500 heat cycles with an expander time of 30 seconds and a time interval of 15 seconds.
Measurement of shear bond strength
This test is a simple evaluation to check dental adhesive bonding. The experimental measurement of the shear bond strength is a useful and necessary test to predict the performance of adhesive systems and their clinical performance. This test is measured by Universal Testing Machine (Instrone), which is often used to assess the ability of bond the restorative and adhesive materials.
The shear force perpendicular to the contact surface of the composite and dentin surface was imposed using the Instron Universal Testing Machine (DARTEC, series HC 10) in the research ward of Imam Khomeini Hospital of Tehran with a loading rate of 0.5 mm/min. After testing the samples by the device and recording the obtained numbers, the shear bond strength was measured in MPa by dividing the forces obtained in terms of Newton by the surface area of the composite and dentin contact area in square millimeters.
(F/A) Bond strength in MPa=force in Newton (F)/Cross section area (A)
Diameter of composite cylinder connected to tooth labial level=4 mm
(A) Cross section area=πr2
Results
In this study, 40 intact anterior primary teeth in 4 groups of 10 were obtained for the present study. After measuring the shear bond strength of resin bonded to the dentin surface of teeth, the resulting numbers were calculated by the cross-sectional area of the bonding segment and calculated using the formula p=F/A based on MPa analyzed. The mean shear strength of the composite bond to the dentin in normal saline group, sodium hypochlorite group, sodium hypochlorite and sodium ascorbate group, and ascorbate sodium group were as much as 31.95 ± 4.90, 12.71 ± 3.04, 28.76 ± 5.34, and 28.57 ± 4.63, respectively (Table 1 and Figure 1).
According to Table 1, the highest bond strength was in normal saline group with a mean as much as 31.95 ± 4.90 and the lowest bond strength was in the sodium hypochlorite group with a mean as much as 12.71 ± 3.04. To compare the shear bond strengths of composite to dentin, Tukey test was used in two groups (Tables 2 and 3).
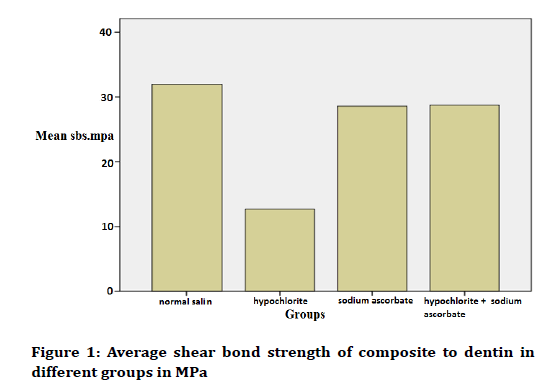
Figure 1. Average shear bond strength of composite to dentin in different groups in MPa
| Groups | Number | Mean | Standard Deviation | Standard Error | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Normal saline | 10 | 31.95 | 4.90 | 1.55 | 24.71 | 38.47 |
| Sodium hypochlorite | 10 | 12.71 | 3.04 | 0.96 | 8.16 | 16.62 |
| Sodium ascorbate | 10 | 28.57 | 4.63 | 1.46 | 22.58 | 37.28 |
| Sodium hypochlorite+Sodium ascorbate | 10 | 28.76 | 5.34 | 1.68 | 23.02 | 38.53 |
Table 1: Central dispersion indicators of shear bond strengths of composite to dentin in different groups.
| Groups | Normal saline | Sodium hypochlorite | Sodium ascorbate | Sodium hypochlorite + Sodium ascorbate |
|---|---|---|---|---|
| Normal saline | - | 19.23 | 3.37 | 3.19 |
| Sodium hypochlorite | - | - | -15.85 | -16.04 |
| Ascorbate Sodium | - | - | - | 0.18 |
Table 2: Comparison of mean values of the shear bond strength of composite to dentin in square style.
| Groups | Normal saline | Sodium hypochlorite | Ascorbate Sodium | Sodium hypochlorite + Sodium ascorbate |
|---|---|---|---|---|
| Normal saline | - | *0.000 | 0.362 | 0.411 |
| Sodium hypochlorite | - | - | *0.000 | *0.000 |
| Ascorbate Sodium | - | - | - | 1.000 |
| *The difference between the two groups is statistically significant. | ||||
Table 3: Comparison of p-values in different groups in square style
The results of this test showed that there was a significant difference between sodium hypochlorite and normal saline (p=0.000) groups. There was a significant difference between sodium hypochlorite and sodium ascorbate alone (p=0.000). There was a significant difference between sodium hypochlorite and sodium ascorbate (p=0.000), but no significant difference was observed between the other groups.
Discussion
Regarding the structural difference between permanent and primary teeth and the absence of a study on the effect of ascorbate sodium on shear bond strength of composite to the dentin of primary teeth and the existence of contradictory results on the effect of sodium hypochlorite on bond strength, this study was conducted to evaluate the effect of Sodium hypochlorite and Sodium Ascorbate on the shear bond strength of the composite to the dentin of primary teeth.
Some studies about comparison of the bond strength of resin materials to the dentin of permanent and primary teeth conclude that the bond strength in primary dentin is less than permanent dentin at the same condition [19,20]. To make the dentin acceptable marginal adherence, at least 17 MPa shear bond strength is required to compensate for the stress caused by polymerization contraction in a composite resin [21]. The optimum bond of resin to dentin is obtained after full monomer penetration into the demineralized dentin [22]. Sodium hypochlorite is a non-specific and disinfectant proteolytic agent used in many dental procedures, such as endodontic treatments, mechanicalchemical removal of caries and dentin bonding techniques [23,24]. In this study, the mean shear bond strength of composite to dentin was as much as 31.95 ± 4.90 in normal saline group as the highest level and 12.71 ± 3.04 in sodium hypochlorite group as the least level. Reducing bond strength after endodontic irrigants with sodium hypochlorite can be attributed to the change in physical and chemical properties of dentin [25]. In addition, sodium hypochlorite decomposes into sodium chloride and oxygen, which the formation of oxygen bubbles in the interior of the dentin and resin prevents from the full penetration of the resin into dentinal tubules [26]. Bahrololoomi et al. stated that the use of sodium hypochlorite leads to durability and the use of sulfic acid salt to improve bonding efficiency [17], which contradicts this study. The reason for this inconsistency can be the different tests performed (shear versus tensile strength), the type of different materials used, the various cutting speed of the device or the use of self-etch adhesives in his study.
Nilavarasan et al. [26] concluded that the use of different concentrations of sodium hypochlorite did not affect the shear bond strength of composite to dentin. This finding contrasts with the present study, the reason for this difference is the use of sodium hypochlorite after dentin etching with phosphoric acid (in the present study, sodium hypochlorite was used before dentin etching) or using bonding with acetone base (one-step plus) in his study. In the present study, single bond was used as an adhesive. This bonding agent has a water-alcohol base. When sodium hypochlorite is used, the strength of the bond decreases due to the low single bond ability to penetrate the spaces created by sodium hypochlorite. Intertobular space created by sodium hypochlorite cannot be filled with large single bond molecules, which itself can be an explanation for reducing the power of the bond when it is used. Mathai et al. stated that the use of sodium hypochlorite reduces the shear bond strength of the composite. It is worth noting that in this study, as in the present study, single bond was used as bonding [27]. Morris in his study showed that rinsing with 5% sodium hypochlorite significantly reduced the bond strength of composite to dentin [10]. Lai et al. also stated that the use of sodium hypochlorite when used with single bond reduces the bond strength of composite to dentin [28]. In the present study, bond strength reduction of single bond was observed, which is consistent with the recent study.
In this research, 10% ascorbate solution was placed on the teeth surface for 2 minutes after applying 5.25% sodium hypochlorite for 1 minute on the teeth surface, which increased the bond strength of composite to dentin and increased the mean bond strength from 12.71 MPa to 24.96 MPa although this amount did not reach the level of normal saline control group. The results of this study were consistent with Moezizadeh et al. [14], Turkun et al. [15] and Gokce et al. [16]. In all of these studies, sodium ascorbate was used after bleaching the enamel with hydrogen peroxide, and there was a marked increase in the bond strength of the composite to the enamel. The results of Vongphan et al. [11] showed that the bond strength of the treated group with 5.25% sodium hypochlorite was lower than the control group, i.e., the water-treated group (30.1 MPa vs. 21.0 MPa) while if the samples were washed with water for 10 minutes, after rinsing with sodium hypochlorite, the bond strength improved slightly (23.1 MPa). In this study, the bond strength increased significantly (40 MPa) after rinsing samples treated with sodium hypochlorite with 10% sodium ascorbate, which was even significantly more than the bond strength of the control group. Contrary to the present research in which the band strength increased, but it was still less than the control group. When the samples were rinsed with water for 10 minutes after treatment with sodium ascorbate, the strength of the band did not differ significantly with the group treated with sodium hypochlorite. The reason for this reduction in bond strength may be attributed to the technical sensitivity of total etching to moisture content of the dentin. The products produced by the reaction of sodium ascorbate and sodium hypochlorite cause a higher bond strength of the group treated with sodium ascorbate, but when water was used after sodium ascorbate, the progress and upgrade of the band has not been accepted [11].
Conclusion
Using 5.25% sodium hypochlorite reduces the shear bond strength of the composite to the primary dentin and the use of 10% sodium ascorbate can reverse the reduction of bond strength. Due to the negative effects of sodium hypochlorite on the bond strength of the restorative materials, other irrigants such as chlorhexidine can be used. Finally, it is recommended to conduct clinical trials to obtain more accurate results and use different concentrations and forms of sodium ascorbate.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- Mirani A. Experimental study of the effect of different color fluids on the dyeing of several types of dental resin composite and dental enamel [Thesis]. Tehran: Shahid Beheshti University of Medical Sciences, Tehran 2010.
- Afzali B, Qasemi A, Jafarzadeh M. Effect of etch time on shear bond strength of a single bond to dentin in-vitro. JDS (SBMU) 2004; 22:302-9.
- Bordin AS, Sefton J, Davies E. In vitro bond strengths of three current dentin adhesives to primary and permanent teeth. Dent Mater 1992; 8:74-8.
- Kosari A, Saraj B, Pasdar N. Shear strength of bonded composite resin to dentin of teeth after laser irradiation of ND: YAG. J Dent (Tehran) 2002; 15:55-46.
- McComb D, Smith DC. A preliminary scanning electron microscopic study of root canals after endodontic procedures. J Endod 1975; 1:238-42.
- Hashemi A. Comparison of IKI, EDTA, 1% sodium hypochlorite solution and 3% sodium hypochlorite solution as an endodontic irrigant in dental teeth [Thesis]. Ahvaz: Jundishapur University of Medical Sciences and Health Services, Ahvaz, Iran 2014.
- Inose S, Murata Y, Sano H, et al. Effect of NaOCl treatment on bond strength between indirect resin core-buildup and dentin. Dent Mater 2002; 21:343-54.
- Tabrizi ZM, Abrishan M, Daneshi M. The effect of smear layer on permeability of human dentinal tubules by dye penetration method. J Mash Dent Sch 2010; 28:22-7.
- Pascon FM, Kantovitz KR, Sacramento PA, et al. Effect of sodium hypochlorite on dentine mechanical properties: A review. J Dent 2009; 12:903-8.
- Morris MD, Lee KW, Agee KA, et al. Effects of sodium hypochlorite and RC-prep on bond strengths of resin cement to endodontic surfaces. JOE 2001; 27:753-7.
- Vongphan N, Senawongse P, Somsiri W, et al. Effects of sodium ascorbate on microtensile bond strength of total-etching adhesive system to NaOCl treated dentine. J Dent 2005; 33:689-95.
- Ari H, Yasar E, BellàS. Effects of NaOCl on bond strengths of resin cements to root canal dentin. JOE 2003; 29:248-51.
- Lai S, Mak Y, Cheung G, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res 2001; 80:1919-24.
- Moezizadeh M, Ghasemi A, Davaran S, et al. Shear bond strength of composite to sodium ascorbate applied on bleached enamel. Beheshti Univ Dent J 2005; 23:138-50.
- Turkun M, Kaya A. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J oral Rehabil 2004; 31:1184-9.
- Gokce B, Çomlekoglu ME, Ozpinar B, et al. Effect of antioxidant treatment on bond strength of a luting resin to bleached enamel. J Dent 2008; 36:780-5.
- Bahrololoomi Z, Dadkhah A, Alemrajabi MS. The effect of Er-Yag laser irradiation and different concentration of sodium hypochlorite on shear bond strength of composite to primary teeth dentin. JLMS 2017; 8:29-35.
- Baghalian A, Nakhjavani Y, Hooshmand T, et al. Microleakage of Er:YAG laser and dental bur prepared cavities in primary teeth restored with different adhesive restorative materials. Laser Med Sci 2013; 28:1453-60.
- Ankyords B, Sefton J, Davis EH. Invitro bond strengths of three current dentin adhesives to primary and permanent teeth. Dent Material 1992; 8:74-8.
- Salma FS, Tao L. Comparison of Glumu bond strength to primary and permanent teeth. Pediatr Dent 1999; 13:163-6.
- GarcÃÂa-Godoy F. Bond strength and interfacial micromorphology of four adhesive systems in primary and permanent molars. ASDC J Dent Child 1998; 65:169-76.
- Vargas MA, Cobb DS, Armstrong SR. Resin-dentin shear bond strength and interfacial ultrastructure with and without a hybrid layer. Oper Dent 1997; 22:159-66.
- Correr G, Puppin-Rontani R, Correr-Sonherti M, et al. Effect of sodium hypochlorite on dentine bonding in primary teeth. J Adhes Dent 2004; 6:307-12.
- Perdiago J, Lopes M, Geraldeli S. Effect of sodium hypochlorite gel on dentin bonding. Dent Mater 2000; 16:311-23.
- Grigoratos D, Knowles J, Ng YL, et al. Effect of exposing dentine to sodium hypochlorite and calcium hydroxide on its flexural strength and elastic modulus. Int Endod J 2001; 34:113-9.
- Nilavarasan N, Hemalatha R, Vijayakumar R, et al. Comparsion of compressive strength among three different intracanal post materials in primary anterior teeth: An invitro study. Eur J Dent 2016; 10:464-8.
- Mathai V, Angelo M, Jaya K, et al. Effect of sodium hypochlorite on shear bond strength using three different adhesive systems: An in-vitro study. IJBIO 2013; 02:637-40.
- Lai SC, Mak YF, Cheung GS, et al. Reversal of compromised bonding to oxidized etched dentin. J Dent Res 2001; 80:1919-24.
Author Info
Razieh Meshki1, Fateme Zarouni2* and Parisa Sarikhani2
1Department of Pediatric Dentistry, School of Dental Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran2Pediatric Dentistry Resident, Department of Pediatric Dentistry, School of Dental Medicine, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
Citation: Razieh Meshki, Fateme Zarouni, Parisa Sarikhani, Effect of Sodium Hypochlorite and Sodium Ascorbate on the Shear Bond Strength of Composite to the Dentin of Primary Teeth , J Res Med Dent Sci, 2019, 7(3): 32-37.
Received: 15-Apr-2019 Accepted: 05-May-2019
