Research - (2020) Advances in Dental Surgery
Effect of Topical Melatonin Application on the Peri-Implant Proximal Bone Level and Cortical Plate Thickness (Pilot Clinical Trial)
Zaid M Yasser*, Ali A Abdulkareem and Saif S Saliem
*Correspondence: Zaid M Yasser, Department of Periodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Introduction: The success rate of dental implant DI is determined by many factors including the maintenance of marginal bone height. Recently, melatonin (MLT) shows a positive effect on bone remodeling, formation, and enhancing bone density. Aims: To investigate the effectiveness of topical MLT application during DI placement on the proximal bone height and cortical plate thickness by using cone-beam computed tomography (CBCT). Materials and Methods: This study was a single-blinded trial following a split-mouth study design with a delayed placement protocol. Selected patients received DI that was placed at contralateral sites that were randomly assigned as “Study” at which 1.2 mg of MLT powder topically applied in the osteotomy site before DI insertion. The other side was the “Control” group where DI placed conventionally. CBCT was taken for the DI at baseline data and after 6 months. Results: For the Control, there was a significant mesial and distal marginal bone reduction (P<0.05) at the end of the trial. While for the Study no significant mesial bone loss was observed; however, the distal bone level showed a significant bone loss (P< 0.05) at the endpoint. Analysis of cortical bone thickness at the buccal and lingual/palatal aspect indicated a significant reduction at the end of the trial in association with the Control. In contrast, no significant differences were observed in association with Study sites. Conclusion: Topically applied melatonin powder showed a positive effect on maintaining the proximal bone level and cortical plate thickness around the dental implant.
Keywords
Dental implant, Melatonin, CBCT
Introduction
Dental implant (DI) placement for replacing missing teeth in completely and partially edentulous patients is an effective and predictable treatment modality. However, failures still a possibility in spite of high implant survival and success rates [1]. Failures of DI can be distributed into early and late failures, depending on whether they occur before (early) or after occlusal loading (late) [2]. Failure of DI that occurs before occlusal load results from a failure to create an intimate bone-implant contact. In this case, bone healing after implant placement is reduced and may be predisposed by many local and systemic factors [2].
The long-term survival of DI depends on its successful osseointegration with the bone. The most implant material select for use in dental applications is titanium (Ti). However, Ti surface properties are not well suitable for connection to the bone. Both surface topography and surface chemistry modifications have led to significant enhancements in the integration of Ti with the bone [3]. Nowadays, attention has been directed towards surface modification of the Ti implant. Several procedures have been suggested to enhance and hasten bone healing using topical treatments. This included the use of bone morphogenetic proteins (BMP), platelet-rich plasma, growth factors, and melatonin (MLT) [4]. MLT has shown a promising role in bone remodeling and formation. MLT is essentially produced and secreted by the pineal gland and other organs. In the oral cavity, MLT shows many effects include free-radical scavenging, antioxidant, and immune-enhancing properties [5]. Additionally, its positive effects are further improved when combined with fibroblasts growth factor-2 [6]. MLT protects the bone by specific mechanisms include its actions on both osteoclasts and osteoblasts. In vitro, MLT prompts the production of type I collagen fibers in human osteoblasts [7]. In addition, MLT increases the bone sialoprotein expression and other protein markers of bone including alkaline phosphatase, osteopontin, and osteocalcin in preosteoblasts, in the result differentiation period of the preosteoblast reduce from 21 to 12 days [8]. Furthermore, MLT down-regulates the receptor activator of nuclear factor kappa-Β ligand (RANKL) which may interfere with the action of osteoclasts and, thereby, preventing bone resorption [9,10].
Based on the previously reviewed literature, we hypothesized the MLT has useful effects when applied topically during implant treatment. MLT potentially could stimulate peri-implant bone formation, reducing proximal bone loss, and enhance the cortical plate thickness around the implant neck.
This trial aimed to investigate the proximal bone height and cortical plate thickness following the topical application of MLT around dental implants. This was achieved by using a cone-beam computed tomography (CBCT) for calculating the mesial and distal bone level around the dental implant and cortical plate thickness (buccal and lingual/palatal) at baseline and after 6 months follow-up.
Materials and Methods
Study design
This study was a randomized clinical trial that was conducted in the Dental Implant Unit, Department of Periodontics, College of Dentistry, the University of Baghdad. The trial was performed after obtaining approval from the ethics committee (Ref. 132619, in 2\12\2019) following the Tokyo and Helsinki declaration of human research. The study was a splitmouth technique, one side served as Control (conventional treatment without MLT) and the Study side (topical application of MLT powder in the implant site).
Inclusion criteria
Patients of both sexes exhibiting good oral hygiene.
Patients with healthy periodontal conditions.
Patients having two missing teeth or more in the maxilla or mandible (1st premolar to 1st molar area) appropriate for the DI replacement.
Bone density ranging between D2 to D3 (1250 Hu - 350 Hu) measured by CBCT according to Misch et al. [11] classification of bone quality.
Exclusion criteria
Any systemic diseases may disturb bone healing such as diabetes mellitus and osteoporosis.
Fully edentulous.
Parafunctional habits.
Smokers.
Patients who were not willing to participate.
The participants were informed about the purpose and the methods used for the research, what their participation in the research entails and what risks, if any, are involved, and their participation was voluntary. If they decide to take part, they asked to sign a consent form, and even that they were still free to withdraw at any time and without giving a reason. All included participants received motivation and oral hygiene instructions then scaling (5-7) days before the surgical treatment.
Surgical procedure
The surgery was performed under local anesthesia (lidocaine hydrochloride 2% with adrenaline 1:80,000 in 2.2 mL) (Septodont, USA) by infiltration or block technique. An extended flap design (full-thickness mucoperiosteal flap) was prepared using surgical blade No. 15 and reflected to expose the implantation site for DI placement.
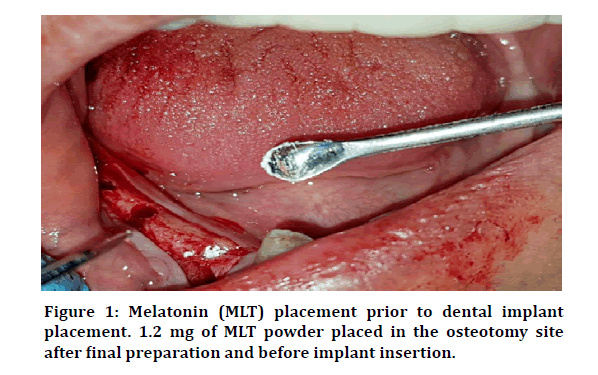
The delayed treatment protocol was followed, according to which the implant placement was done in the healed site at least 6 months after tooth extraction. After the elevation of the flap, the proposed DI site was exposed then the bony bed was prepared. The conventional drilling technique followed in sequence until getting the requested final drill size according to the manufacturer’s instructions of the DI system (Dentuim Co, Korea). For the study group, 1.2 mg of MLT was placed in the osteotomy site, by small spatula, before the placement of the DI (Figure 1). According to Cutando et al. [12], this dose of MLT per DI is sufficient to improve the osseointegration of DI and reduce the marginal bone loss.
Figure 1: Melatonin (MLT) placement prior to dental implant placement. 1.2 mg of MLT powder placed in the osteotomy site after final preparation and before implant insertion.
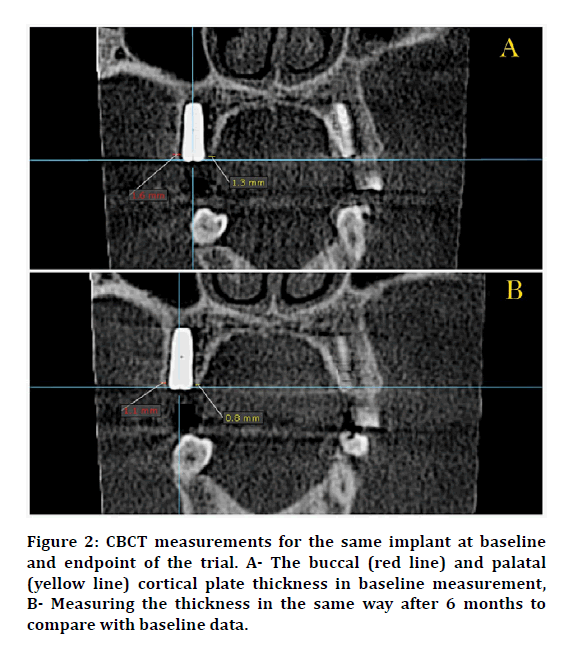
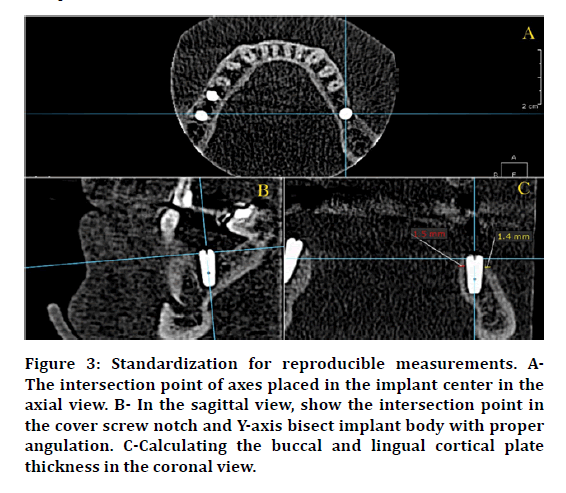
After DI insertion, the flap was sutured back in place using an interrupted suturing technique with a (4/0) black silk non-absorbable suture material in the delayed treatment protocols. Immediately after the surgery, CBCT was taken to show the position of the implant, the implant to other dentition relationships, vital structures, measuring the mesial and distal bone level in the sagittal view, and to measure the buccal and lingual \ palatal cortical plate thickness as baseline data. After 6 months, another CBCT was taken to observe the amount of the proximal bone loss that occurred. Proximal bone level and cortical plate thickness were measured in the same way as in baseline measurement (Figure 2). To notice the same slice in the CBCT and to avoid any mistakes that may influence in the reading of the slice, the intersection point of axes must be centered in the cover screw notch and used it as a reference point (Figure 3).
Figure 2: CBCT measurements for the same implant at baseline and endpoint of the trial. A- The buccal (red line) and palatal (yellow line) cortical plate thickness in baseline measurement, B- Measuring the thickness in the same way after 6 months to compare with baseline data.
Figure 3: Standardization for reproducible measurements. AThe intersection point of axes placed in the implant center in the axial view. B- In the sagittal view, show the intersection point in the cover screw notch and Y-axis bisect implant body with proper angulation. C-Calculating the buccal and lingual cortical plate thickness in the coronal view.
Results
A total of 8 patients, 4 males, and 4 females were included in this study. Their age ranged between 40-55 years (Table 1). They received 26 dental implants that were placed using a split-mouth study design with a delayed placement protocol.
| Variables | |
|---|---|
| Age (years) | |
| Mean± SD | 47± 4.98 |
| Minimum | 42 |
| Maximum | 55 |
| Range | 13 |
| Median | 45 |
| Gender§ | |
| Male | 4, 50 |
| Female | 4, 50 |
| Total | 8, 100 |
§ Frequency, percent
Table 1: Demographic characteristics of the study population.
In the Control, the mean height of alveolar bone at the mesial side at baseline (day 0) was equal to 10.41 mm while after 6 months at the end of the trial equal to 9.50 mm. Statistically, this indicated a significant mesial marginal bone loss in intra-Control comparison (P<0.001) (Table 2). The intra-Study comparison showed no significant bone loss. In detail, the mean bone level in baseline (day 0) was equal to 10.01 mm and after 6 months the mean was 9.87 mm (Table 2). The inter-groups comparison (Control vs. Study) suggested no significant reduction in mesial bone level when comparing the two groups in each time point (baseline and after 6 months) (Table 2). For the intra-Control group comparison, the results suggested a significant distal bone level reduction (P =0.004). Similarly, the intra-Study group comparison, according to distal bone level, at baseline (mean =9.63 mm) and the end of the trial (mean =8.93 mm) showed a significant bone loss (P=0.014) (Table 2). Intergroups comparison between the Control and Study in each time point (baseline and after 6 months) showed no significant distal bone level reduction (Table 2).
| Mesial | Baseline | Endpoint§ | Comparisons | P-value* | |
|---|---|---|---|---|---|
| Control | Mean | 10.41 | 9.5 | Baseline vs Endpoint | <0.001 |
| ± SD | 2.079 | 2.402 | |||
| ± SE | 0.576 | 0.666 | |||
| Study | Mean | 10.01 | 9.87 | Baseline vs Endpoint | 0.395 (NS) |
| ± SD | 1.814 | 2.049 | |||
| ± SE | 0.503 | 0.568 | |||
| Control vs study | P-value** | ||||
| Baseline | 0.606 | ||||
| Endpoint | 0.671 | ||||
| Distal | |||||
| Control | Mean | 9.79 | 9.14 | Baseline vs Endpoint | 0.004 |
| ± SD | 1.85 | 2.043 | |||
| ± SE | 0.513 | 0.566 | |||
| Study | Mean | 9.63 | 8.93 | Baseline vs Endpoint | 0.014 |
| ± SD | 1.806 | 1.96 | |||
| ± SE | 0.5 | 0.543 | |||
| Control vs study | P-value** | ||||
| Baseline | 0.824 | ||||
| Endpoint | 0.786 | ||||
§ 6-month post-implant placement
* Significance at P< 0.05 by using paired t-test
** Significance at P< 0.05 by using unpaired t-test
NS: non-significant
Table 2: Inter- and intragroup comparison of CBCT measurements (mm) for the proximal alveolar bone level at baseline and end of the trial.
For cortical bone thickness at the buccal aspect, the results indicated a significant reduction (P-value=0.010) at the end of the trial in association with the Control. In contrast, no significant differences were observed at the same site in the Study sites (Table 3). However, buccal cortical plate thickness was not significantly differed between the Study and Control at the endpoint (Table 3). The same inter- and the intra-group pattern was observed in association with the measurements of the cortical plate thickness (Table 3).
| Buccal | Baseline | Endpoint§ | Comparisons | P-value* | |
|---|---|---|---|---|---|
| Control | Mean | 1.262 | 1.077 | Baseline vs Endpoint | 0.01 |
| ± SD | 0.317 | 0.245 | |||
| ± SE | 0.088 | 0.068 | |||
| Study | Mean | 1.331 | 1.215 | Baseline vs Endpoint | 0.054 |
| ± SD | 0.464 | 0.387 | |||
| ± SE | 0.128 | 0.107 | |||
| Control vs study | P-value** | ||||
| Baseline | 0.661 | ||||
| Endpoint | 0.287 | ||||
| Lingual/palatal | Baseline | Endpoint§ | Comparisons | P-value* | |
| Control | Mean | 1.392 | 1.185 | Baseline vs Endpoint | <0.001 |
| ± SD | 0.409 | 0.389 | |||
| ± SE | 0.113 | 0.107 | |||
| Study | Mean | 1.3 | 1.254 | Baseline vs Endpoint | 0.654 |
| ± SD | 0.489 | 0.366 | |||
| ± SE | 0.135 | 0.101 | |||
| Control vs study | P-value** | ||||
| Baseline | 0.607 | ||||
| Endpoint | 0.645 | ||||
§ 6-month post-implant placement
* Significance at P< 0.05 by using paired t-test
** Significance at P< 0.05 by using paired t-test
NS: non-significant
Table 3: Inter- and intragroup comparison of CBCT measurements (mm) for the buccal and lingual/palatal cortical plate thickness at baseline and end of the trial.
Discussion
In the present study, we evaluated the effect of topical application of MLT powder in the proximal bone height around the DI and thickness of the buccal and lingual cortical plate after 6 months from endosseous implant insertion in the splitmouth study. Sites treated with MLT showed a significantly lower reduction in the proximal bone level and cortical plate thickness at the end of the trial.
The successful clinical outcomes of the most osseointegrated DI depend on reaching the bone remodeling to the steady state. The marginal bone loss (MBL) is the main clinical outcome of the disproportion between bone formation and loss. One of the most important criteria for describing implant success is marginal bone stability around dental implants [13]. Several materials have been used to improve periimplant bone quality/quantity such as growth factors, BMP [14], and recently hormones, such as growth hormone and MLT [3]. One of the important action of MLT is the bone cells formation, in vitro, MLT shows positive stimulus in the proliferation and differentiation of human osteoblasts as well as in the synthesis of type I collagen and other proteins of the bone matrix [15,16]. MLT stimulates the differentiation of the preosteoblast cells by reducing the period from 21 days to 12 days [16].
According to CBCT measurement in our study, the bone level in the mesial aspect in the Control was significantly less than in the Study. Additionally, we observed that bone loss in the mesial aspect at the end of the study was significantly higher in the Control than the MLT group. In the distal aspect, a significant bone loss in both the Control and Study groups. The same outcome was confirmed by Hazzaa et al. [17] who demonstrated significantly less MBL at sites that received MLT with the autogenous bone graft (ABG), after 6 and 9 months, as compared with the control group (only ABG). Besides, our results could be further supported by previous findings that observed osteoclasts-inhibiting potential of MLT [18].
CBCT readings about the thickness of the cortical plate at the buccal and lingual/palatal aspects revealed a significant reduction in plate thickness in the Control while in the MLT group the cortical thickness was better preserved at the end of the trail. This is in agreement with Guardia, Gómez–Moreno (18) who have shown in a previous study that bone-implant contact (BIC) and total Peri-implant bone area at 5 and 8 weeks were greater in the implant with MLT than in the control group. Our results can be clarified by the biological effects of MLT which significantly increase the formation of bone cells. Several studies have indicated that MLT boosts the proliferation and differentiation of human osteoblasts in vitro, as well as the synthesis of type I collagen and other proteins of the bone matrix [15,16]. In addition, MLT reduces the expression of RANK in osteoblasts and RANK receptor in osteoclasts while increasing osteoprotegerin, eventually halting the differentiation and activation of the osteoclasts [19]. This suggests that MLT potentially reduces bone loss and enhances bone quantity by downregulating RANK-mediated osteoclast. Moreover. MLT could play role in maintaining bone density and inhibiting bone resorption by its action in the osteoclast lacuna together with antioxidant properties and its ability to neutralize reactive oxygen species [20].
The main limitation of the current trial was the limited sample size due to COVID-19 pandemic restrictions. Additionally, the level of bone healing biomarkers, antioxidative effect of MLT in the peri-implant sulcular fluid were not measured due to limited resources. Therefore, further studies on a larger scale that include other biochemical assays are recommended. Despite limitations, the current study provided promising results about using MLT in combination with DI procedure. However, the current results represented outcomes of a pilot study that should not be considered conclusive until confirmed by studies on a larger number of populations.
Conclusion
Our results suggested that the melatonin powder applied topically in the osteotomy site at the time of implant placement was related with:
Less proximal bone loss, which is an indicator of improved osseointegration.
Significantly maintaining the cortical plate thickness after 6 months of post-DI placement in association with Study sites as compared to Control.
References
- Chrcanovic B, Albrektsson T, Wennerberg A. Reasons for failures of oral implants. J Oral Rehab 2014; 41:443-476.
- Alsaadi G, Quirynen M, Komárek A, et al. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol 2007; 34:610-617.
- Muñoz F, López–Peña M, Miño N, Gómez–Moreno G, Guardia J, Cutando A. Topical application of melatonin and growth hormone accelerates bone healing around dental implants in dogs. Clinical implant dentistry and related research. 2012;14(2):226-35.
- Zechner W, Tangl S, Tepper G, et al. Influence of platelet-rich plasma on osseous healing of dental implants: A histologic and histomorphometric study in minipigs. Int J Oral Maxillofac Implants 2003; 18.
- Cutando A, Gómez–Moreno G, Arana C, Acuña–Castroviejo D, et al. Melatonin: Potential functions in the oral cavity. J Periodontol 2007; 78:1094-1102.
- Takechi M, Tatehara S, Satomura K, et al. Effect of FGF-2 and melatonin on implant bone healing: a histomorphometric study. J Materials Sci 2008; 19:2949-2952.
- Nakade O, Koyama H, Ariji H, et al. Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J Pineal Res 1999; 27:106-110.
- Satomura K, Tobiume S, Tokuyama R, et al. Melatonin at pharmacological doses enhances human osteoblastic differentiation in vitro and promotes mouse cortical bone formation in vivo. J Pineal Res 2007; 42:231-239.
- Boyce BF, Schwarz EM, Xing L. Osteoclast precursors: Cytokine-stimulated immunomodulators of inflammatory bone disease. Current Opinion Rheumatol 2006; 18:427-432.
- Suzuki N, Hattori A. Melatonin suppresses osteoclastic and osteoblastic activities in the scales of goldfish. J Pineal Res 2002; 33:253-258.
- Misch CE. ARABIC-Contemporary implant dentistry: Elsevier Health Sciences 2007.
- Cutando A, Gómez–Moreno G, Arana C, et al. Melatonin stimulates osteointegration of dental implants. Pineal Res 2008; 45:174-179.
- Trindade R, Albrektsson T, Tengvall P, et al. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Related Res 2016; 18:192-203.
- Luo T, Zhang W, Shi B, et al. Enhanced bone regeneration around dental implant with bone morphogenetic protein 2 gene and vascular endothelial growth factor protein delivery. Clin Oral Implants Res 2012; 23:467-473.
- Radio NM, Doctor JS, Witt–Enderby PA. Melatonin enhances alkaline phosphatase activity in differentiating human adult mesenchymal stem cells grown in osteogenic medium via MT2 melatonin receptors and the MEK/ERK (1/2) signaling cascade. J Pineal Res 2006; 40:332-342.
- Roth JA, Kim BG, Lin WL, et al. Melatonin promotes osteoblast differentiation and bone formation. J Bio Chem 1999; 274:22041-22047.
- Hazzaa HH, El–Kilani NS, Elsayed SAE, et al. Evaluation of immediate implants augmented with autogenous bone/melatonin composite graft in the esthetic zone: a randomized controlled trial. J Prosthodont 2019; 28:e637-e42.
- Guardia J, Gómez–Moreno G, Ferrera MJ, et al. Evaluation of effects of topic melatonin on implant surface at 5 and 8 weeks in Beagle dogs. Clin Implant Dent Related Res 2011; 13:262-68.
- Nakano M, Ikegame M, Igarashi-Migitaka J, et al. Suppressive effect of melatonin on osteoclast function via osteocyte calcitonin. J Endocrinol 2019; 242:13-23.
- Cardinali DP, Ladizesky MG, Boggio V, et al. Melatonin effects on bone: Experimental facts and clinical perspectives. J Pineal Res 2003; 34:81-87.
Author Info
Zaid M Yasser*, Ali A Abdulkareem and Saif S Saliem
Department of Periodontics, College of Dentistry, University of Baghdad, Baghdad, Iraq1Iraq
Citation: Dina Hamid Obaid, Dhiaa Jaafar AL-Dabagh, Israa Salman Jassim, Assessment of Friction Among Nickel Free Orthodontic Brackets and Archwires Combinations in Wet Condition (An In-vitro Comparative Study), J Res Med Dent Sci, 2020, 8 (7): 394-399.
Received: 21-Oct-2020 Accepted: 10-Nov-2020 Published: 17-Nov-2020