Research - (2019) Volume 7, Issue 3
Effects of Red Guava Juice on Hemoglobin and Hematocrit Levels in Female Adolescent Students with Anemia
Mega1, Hidayat Wijayanegara1, Siti Sugih Hartiningsih1*, Menizibeya O Welcome2 and Senol Dane2
*Correspondence: Siti Sugih Hartiningsih, Dharma Husada Institute of Health Sciences, Indonesia, Email:
Abstract
Background: Anemia is a major public health problem. Iron deficiency anemia is the most common form of anemia due to malnutrition especially in developing countries. Adolescents are one of the major groups of risk for anemia. The condition can lead to impaired brain functions resulting to a decrease in learning ability and academic performance. Medicinal foods, in particular, red guava, have been used for decades to treat several ailments of humans. There is paucity of human data on the effects of red guava on anemic conditions. The purpose of this study was to compare the effects of red guava juice on hemoglobin and hematocrit levels with iron supplementation in adolescent female students with anemia.
Methods: The study was approved by the Ethical Committee of the Dharma Husada Institute of Health Sciences, Bandung, Indonesia. The study was conducted among high school students of Tomo Secondary School, Sumedang, Bandung, Indonesia. Out of 236 female adolescent students, a total of 32 (15-18 years, mean age ± standard deviation, 16.45 ± 3.12) had iron deficiency anemia. The anemic students were randomized into two groups: red guava group (n=16) and iron supplementation group (n=16).
Results: Both red guava and iron supplementations resulted in a significant increase in both hemoglobin and hematocrit levels. The effectiveness of both was not different each other.
Conclusion: The results of this study suggest that red guava juice was effective as iron supplementation in increasing hemoglobin and hematocrit levels.
Keywords
Iron deficiency anemia, Hematocrit, Hemoglobin, Red guava juice
Introduction
Anemia still attracts the attention of researchers and policy makers worldwide due to the high prevalence and the resulting menace posed by the condition [1,2]. Anemia is a major public health problem with a global prevalence of about 2 billion [3,4]. Adolescents are one of the major risk groups for anemia. The incidence of anemia among adolescents is higher in developing countries (27%), compared to only 6% in developed countries [5]. The prevalence of anemia in Indonesia is reported to be around 16%-39% [2]. However, Barkley et al. identified substantial changes in trends of anemia over time in Indonesia, hence the need for continuous monitoring of the prevalence of anemia [2]. Unfortunately, there is lack of data on the prevalence of anemia in several regions of Indonesia, including Tomo Secondary School, Sumedang, Indonesia.
The most common type of anemia is iron deficiency anemia, occurring mainly due to nutritional deficiency [6]. Iron deficiency anemia is the major type of anemia during the adolescent period [4]. In addition to malnutrition and accelerated growth, hormonal changes and onset of menstruation, the condition is believed to be worse in female adolescents [5]. Due to the importance of iron in almost all organs and tissues of the body, deficiency of this micronutrient may lead to impairment in perception, learning and memory, consequently resulting to decrease in academic performance among students [5].
The problem of iron deficiency anemia in students is further worsened by the eating habits, hence the need to monitor and where necessary carry out intervention to tackle the problem [2]. Both iron supplementation and medical plant products have been used to address the problem of anemia in different settings [7]. Certain food items as well as medicinal plant products contain considerable amount of iron [8]. Red guava (Psidium guajava Linn, family: Myrtaceae), popularly known as guava, is a medicinal plant found in tropical and subtropical countries [9,10]. Guava trees have been grown by many other countries, thus allowing production around the world [11]. Teas, infusions, and decoctions prepared from the leaves, root, bark, seeds, fruits, and flowers are safe for oral and topical application and used for treatment purposes [9,12]. The fruit serves as food [9].
Though, traditionally, preparations of the leaves have been used in folk medicine in several countries, mainly as anti-diarrheal ant- dehydration remedy [13], over the past decades, accumulating data have shown that red guava is effective in addressing different infections, including gastroenteritis and dysentery [9,14-17], act as immunostimulant [18], anti-hyperglycemic [19], antiapoptotic [18,20], chemopreventive, chemotherapeutic [21], hepatoprotective [22], antioxidant [23], anti-allergy, cardioactive, and antinociceptive agent [24]. An animal study conducted by Uboh et al. revealed that red guava extract significantly increased hemotocrit and hemoglobin levels [25]. However, there is lack of human data on the effects of red guava on hematocrit and hemoglobin levels. No study has specifically addressed possible effects of red guava on the levels of hemoglobin and hematocrit in patients with anemia.
The purpose of this study was to investigate the effects of red guava juice on the levels of hemoglobin and hematocrit in female students with iron deficiency anemia.
Materials and Methods
Ethical statement and clearance
The study was approved by the Ethical Committee of Dharma Husada Institute of Health Sciences, Bandung, Indonesia (ethical clearance number: 010/STIKes- DHB/ Sket/PSKBS2/X/2017).
Participants
Before the commencement of the study, 236 female adolescent female students of Tomo Secondary School, Sumedang, Bandung, Indonesia, were approached to volunteer for the study after the aims and objectives, study methodology, expected risks and benefits had been thoroughly explained to them.
Students were free to discontinue or withdraw from the study at any given point. A total of 228 who volunteered for the study were screened for anemia. Of them, 43 students who had positive signs of anemia on the basis of preliminary assessment were selected for hematological investigation. However, 33 volunteered for the hematological investigation.
Based on the results of hematology, one of the 33 participants was excluded as the participant did not have iron deficiency anemia and was referred to the nearest Health Center further for diagnosis and treatment. A total of 32 (range 15-18 years, mean age ± standard deviation, 16.45 ± 3.12; weight 42-56 Kg, average 47.38 ± 2.14 Kg) had iron deficiency anemia according to hemoglobin, hematocrit and serum ferritin levels.
The anemic students were randomized into two groups: red guava group (n=16) and iron supplementation group (n=16). Their age range was from 15 years to 18 years (mean age ± standard deviation, 16.45 ± 3.12). There was no age and body weight difference between the two groups.
Inclusion criteria
1. Willingness to participate.
2. The presence of anemia
3. Absence of any other health problems based on recent medical examination.
Exclusion criteria
1. Unwillingness to participate in the study.
2. Presence of other health problems other than anemia such a metabolic, cardiac or renal disease, which may affect hemoglobin or hematocrit based on anamnesis.
Procedure
This study was conducted for 2 weeks i.e., from December 2017-January 2018. After approval of the experimental protocol, the aims and objectives of the study were explicitly explained to the participants before commencing the experiment.
All the volunteers signed an informed consent before starting the experimental session. The first phase of the study was carried out by checking for conjunctiva pallor and capillary refill. Students who had pallor and a delayed capillary refill (normal 1-2 seconds) were brought to the local health laboratory for hemoglobin and hematocrit analysis. The 32 students reported after 12 h overnight fast and 4 ml venous blood sample was collected for analysis. All students were told to completely abstain from iron rich foods such as cow milk, meat, eggs, oranges, beans, peas, and strawberries. The participants were randomized by assigning random numbers generated using Excel sheet, the list of participants was rearranged according to the random numbers. 16 students each were assigned in each group. For duration of 2 weeks, one group received red guava juice and the other received iron tablet daily. They were given a list of foods with high iron content (including meat, liver and spinach) and advised not to consume the stated foods.
Obtention and preparation of red guava juice
Fresh guava fruits without lesions induced mechanically or by pathogens were purchased locally from Saluyu Jaya village in Majalengka sub-district, Bandung, Indonesia. The fruits were identified and authenticated by an Agriculturist from the Food Crops and Horticulture Services of West Java Province Government, and the voucher specimen was deposited in the herbarium (184/B. JmB. BR. IV/3.2017).
The fruits were prepared according to the following recommendations [12] with modifications. The fruits were thoroughly washed in running water, kept in a 0.1% sodium hypochlorite solution for 1 h, then washed in distilled water. The fruits were ground in a blender (Maspion group, Kembang Jepun 38-40, Surabaya 60162, East Java, Indonesia) and subjected to manual filtration. The resulting juice was stored in perfect condition under refrigeration at -25°C before administration.
Supplementation of red guava juice
Sixteen participants included in group A were supplemented with 100 ml of red guava juice everyday under direct supervision for 2 weeks. The quantity of red guava juice was based on the calculation that each 100 ml contains about 0.69 mg of iron (the quantity required by adolescent females is about 0.7 mg-0.9 mg of iron per day at early adolescent stage to 2.2 mg iron per day or even more in heavily menstruating adolescents [26].
Iron supplementation
All participants from the iron supplementation group received a daily dose of ferrous sulfate 300mg, which is equivalent to 60 mg of elemental iron (i.e., 300 × 0.2).
Identification of anemia
Determination of conjunctival pallor and capillary refill time
Conjunctival pallor and capillary refill time were used as measures for screening. Conjunctival pallor and capillary refill time were determined as earlier reported [27]. Conjunctival pallor or capillary refill time is recommended for anemia screening [27].
Collection of blood samples
A maximum of 4 ml of venous blood samples were collected from the antecubital fossa by using aseptic methods, and dispensed into EDTA tubes for hematology for determination of hemoglobin and hematocrit. Sample tubes were obtained from Becton Dickinson (Plymouth, United Kingdom). Blood samples collected in EDTA tubes were stored and transported in cold styrofoam boxes and analyzed within 4 h of collection. Blood was allowed to clot at room temperature (25°C) and was centrifuged at 3000 × g for 15 min [28].
Determination of hemoglobin, hematocrit and serum ferritin levels
Hemoglobin, hematocrit, were measured by using validated MSLAB07 plus Hematology Analyzer (Guangzhou MeCan Medical Limited, Guangzhou, Guangdong, China). Reagents, calibrators, and controls were obtained from the instrument manufacturer. Analysis of samples was performed within 8 h of blood draw. Automated determination of hemoglobin, hematocrit levels were performed according to the manufacturer’s specifications.
The hemoglobin cut-off point indicating anemia was done according the World Health Organization. A value less than 12 g/dl or 120 g/l for females (aged 12 years-18 years) was considered as anemia according [25]. Hematocrit was considered to be abnormal at values <0.36 for females [28].
Serum samples for measurement of ferritin were stored at -70°C until they were sent to the Biochemical Laboratory of the Health Center at Sumedang, Bandung, Indonesia for analysis. Ferritin concentration was measured by ELISA technique as previously described [29]. Iron deficiency was defined as ferritin levels <12 μg/l [30]. All participants with iron deficiency had serum ferritin levels lower than <12 μg/l.
Statistical analysis
The SPSS software (version 18.0 for Windows) was used for statistical data analysis. Results are expressed as mean ± standard deviation (SD) as well as in percentages (%). The pattern of data distribution was evaluated by Kolmogorov Smirnov test. Repeated Measures Test in General Linear Model was used for comparison of results of hemoglobin and hematocrit before and after (two measures: 7th and 14th days) therapy. Differences were considered statistically significant at p<0.05.
Results
There were no statistically significant differences in the pretest values of hemoglobin and hematocrit levels between the red guava group and iron supplementation group. Both red guava and iron supplementation in the female students led to increase in both hemoglobin and hematocrit levels (p=0.00) (Figures 1-4). However, there was no statistically significant differences in the values of posttest hematocrit and hemoglobin values between the red guava group and iron supplementation group (F=2.57, p=0.1).
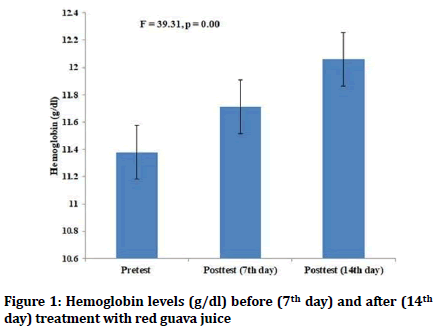
Figure 1: Hemoglobin levels (g/dl) before (7th day) and after (14th day) treatment with red guava juice
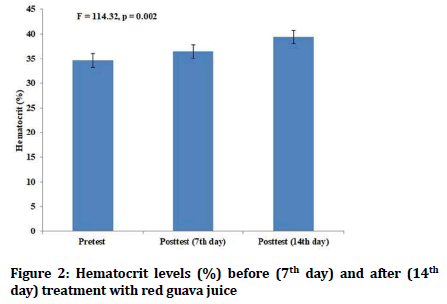
Figure 2: Hematocrit levels (%) before (7th day) and after (14th day) treatment with red guava juice
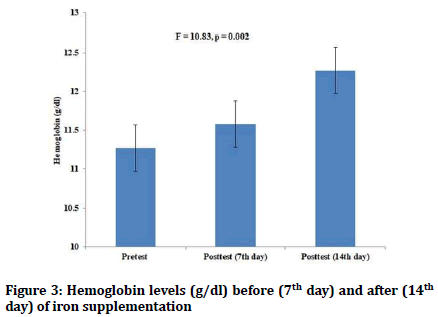
Figure 3: Hemoglobin levels (g/dl) before (7th day) and after (14th day) of iron supplementation
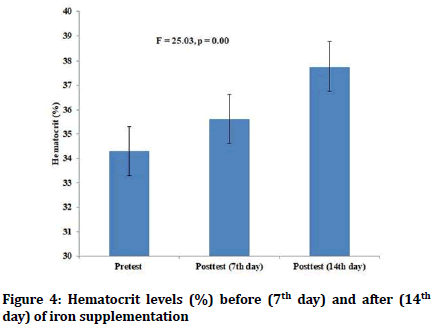
Figure 4: Hematocrit levels (%) before (7th day) and after (14th day) of iron supplementation
Discussion
The use of medical plants for food and treatment is an integral part of the cultures in many parts of the world [31]. These cultures promote health and quality of life with therapies based on the use of natural products [32]. Given that plants have been widely used as herbal medicines, several approaches are now being carried out to discover new bioactive compounds from natural sources [33]. The results of this study suggest that a 2- week supplementation with red guava juice can be effective is treating iron deficiency anemia, which accounts for about 50% of all anemias. Iron deficiency anemia is worse in tropical and subtropical countries due to endemicity of malaria and other parasitic infections [25]. Interestingly, the effects of red guava juice were comparative with those of iron supplementation group. Consequently, red guava juice can be used in place of iron supplementation because it is easier to produce and cheaper. Red guava contains a substantial quantity of iron. The iron content of this medicinal plant is estimated to be about 6.91 mg/kg or equal to 0.69 mg/100 g, which is greater than the iron content in meat (2 mg/kg or equal to 0.2 mg/100 g of iron), liver (5 mg/kg or equal to 0.5 mg/100 g of iron) [34-36]. Although different parts of this plant have been used traditionally to treat many diseases including bleeding gums [25], no empirical investigation has been conducted in humans on the effects of red guava on hematological indices of anemia.
The fact that no significant difference was observed between the iron supplementation group who took 300 mg of ferrous sulfate (60 mg of elemental iron) and the red guava group possibly, suggests some differences in intestinal absorption of iron in different sources. Though 60 mg of elemental iron is relatively high, there are currently many recommendations on oral iron dosage, which vary with age, gender, and physiological states, and may range from 8 mg to 60 mg per day [37-42]. Ingestion of iron within a period of two weeks successfully treated the observed anemia in the volunteers. Indeed iron is absorbed within 24 h-48 h following ingestion [43]. Incorporation of elemental iron into hemoglobin occurs within 4-7 days, but may be as early as 2-3 days, depending on dietary and other factors [44,45]. Iron absorption may even occur within a shorter period of 1 h-6 h depending on the time after food ingestion or fasting [46]. However, Goodnough et al. noted that in patients with iron deficiency about 50% of “intravenous iron” is incorporated into hemoglobin in 3-4 weeks [47]. The reasons for these discrepancies in data are not exactly clear. But it may be due to differences in methodological approaches and peculiarities in intestinal absorption, transport and turnover rate of iron/ hemoglobin in different individuals as well as the presence of diseases that affect iron metabolism and turnover.
The majority of works on the medicinal importance of red guava has been focused on the leaves, which is traditionally used in folk medicine to treat a couple of human diseases including diabetes mellitus, hypertension, infections (gastrointestinal, respiratory, dental, cutaneous), cancer, menstrual problems, pain, hepatic problems among others [48,49].
However, the fruit is the most palatable portion of red guava. Thus the fruit is the most important part of red guava [12]. From the above, the plant has substantial economic importance, in addition to its taste and flavor [12]. Apart from our study, the effects of guava on indices of anemia have not been explored empirically in humans.
Though the mechanisms of action of red guava is not exactly clear, several studies performed around the globe have shown that the medicinal plant contains tens of molecules including over 60 types of compounds: alkaloids, flavonoids, glycosides, polyphenols, saponins [25], anthocyanins, carotenoids, essential oils, fatty acids, lectins, tannins, triterpenes, vitamin C [25], ellagitannins, proanthocyanidins, dihydrochalcones, anthocyanidins, stilbenes, acetophenones, benzophenones, phlorizin, nothofagin, astringin, chrysin-C-glucoside, valoneic acid, bilactone, cinnamoyl-glucoside, dimethoxycinnamoylhexosides [50], gallic acid, epigallocatechin gallate, syringic acid, o-coumaric acid, resveratrol, quercetin, catechin [13], gallic acid, caffeic acid, guaijaverin, carotenoids, triterpenoids [13,24], and quercetin [25]. The role of red guava juice in mediating increase in hemoglobin and hematocrit levels may in part be due to its pleiotropic activities including its antioxidant action. Red guava was shown to suppress inflammatory molecules such as iNOS and NF-κB through activation of PPARγ [10,50].
Conclusion
Red guava juice was effective in increasing hemoglobin and hematocrit levels of female students with anemia. The effects of red guava on hemoglobin and hematocrit levels were comparable to the effects achieved upon administration of iron supplementation. Red guava is a very cheap fruit that even the less privileged people can acquire it easily. Therefore, this fruit juice can be effective not only in managing inflammatory, oxidative states, but also, treat iron deficiency anemia.
Acknowledgement
We acknowledge Dr. HJ. Suryani Soepardan, Chairman of Dharma Husada, Bandung; Principal of Tomo Secondary School, Sumedang, Indonesia, and their co-workers who helped at one or more phases of the study.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
References
- World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization, Vitamin and Mineral Nutrition Information System 2011.
- Barkley JS, Kendrick KL, Codling K, et al. Anaemia prevalence over time in Indonesia: estimates from the 1997, 2000, and 2008 Indonesia family life surveys. Asia Pac J Clin Nutr 2015; 24:452-5.
- Pasricha SR. Anemia: A comprehensive global estimate. Blood 2014; 123:611-2.
- Wang M. Iron deficiency and other types of anemia in infants and children. Am Fam Physician 2016; 93:270-8.
- Balcı YI, Karabulut M, Gürses D, et al. Prevalence and risk factors of anemia among adolescents in Denizli, Turkey. Iran J Pediatr 2012; 22:77-81.
- Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123:615-24.
- Trumbo P, Yates AA, Schlicker S, et al. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Acad Nutr Diet 2001; 101:294.
- US Department of Agriculture, Agricultural Research Service. USDA national nutrient database for standard reference. Nutrient Data Laboratory 2013.
- Morais-Braga MF, Carneiro JN, Machado AJ, et al. Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. J Ethnopharmacol 2016; 194:1140-52.
- Li PY, Hsu CC, Yin MC, et al. Protective effects of red guava on inflammation and oxidative stress in streptozotocin-induced diabetic mice. Molecules 2015; 20:22341-50.
- Salazar DM, Melgarejo P, Martínez R, et al. Phenological stages of the guava tree (Psidium guajava L.). Sci Hortic 2006; 108:157-61.
- Simão AA, Marques TR, Marcussi S, et al. Aqueous extract of Psidium guajava leaves: phenolic compounds and inhibitory potential on digestive enzymes. An Acad Bras Cienc 2017; 89:2155-65.
- Seo J, Lee S, Elam ML, et al. Study to find the best extraction solvent for use with guava leaves (Psidium guajava L.) for high antioxidant efficacy. Food Sci Nutr 2014; 2:174-80.
- Adeyemi OS, Akanji MA. Biochemical changes in the kidney and liver of rats following administration of ethanolic extract of Psidium guajava leaves. Hum Exp Toxicol 2011; 30:1266-74.
- Gutiérrez RMP, Mitchell S, Solis RV. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 2008; 117:1-27.
- Shruthi SD, Roshan A, Sharma S, et al. A review on the medicinal plant Psidium guajava Linn. (Myrtaceae). J. Drug Deliv Ther 2013; 3:162-8.
- Morais-Braga MFB, Carneiro JNP, Machado AJT, et al. Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. J Ethnopharmacol 2016; 194:1140-52.
- Laily N, Kusumaningtyas RW, Sukarti I, et al. The potency of guava psidium guajava (l.) leaves as a functional immunostimulatory ingredient. Procedia Chem 2015; 14:301-7.
- Kumari S, Rakavi R, Mangaraj M. Effect of guava in blood glucose and lipid profile in healthy human subjects: A randomized controlled study. J Clin Diagn Res 2016; 10: BC04-7.
- Dos Santos RC, Ombredane AS, Souza JMT, et al. Lycopene-rich extract from red guava (Psidium guajava L.) displays cytotoxic effect against human breast adenocarcinoma cell line MCF-7 via an apoptotic-like pathway. Food Res Int 2018; 105:184-96.
- Chen KC, Peng CC, Chiu WT, et al. Action mechanism and signal pathways of Psidium guajava L. aqueous extract in killing prostate cancer LNCaP cells. Nutr Cancer 2010; 62:260-70.
- Roy CK, Das AK. Comparative evaluation of different extracts of leaves of Psidium guajava Linn. for hepatoprotective activity. Pak J Pharm Sci 2010; 23:15-20.
- Ravi K, Divyashree P. Psidium guajava: A review on its potential as an adjunct in treating periodontal disease. Pharmacogn Rev 2014; 8:96-100.
- Gutiérrez RM, Mitchell S, Solis RV. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 2008; 117:1-27.
- Uboh FE, Okon IE, Ekong MB. Effect of aqueous extract of psidium guajava leaves on liver enzymes, histological integrity and hematological indices in rats. Gastroenterology Res 2010; 3:32-8.
- Beard JL. Iron requirements in adolescent females. J Nutr 2000; 130:440S-2S.
- Sheth TN, Choudhry NK, Bowes M, et al. The relation of conjunctival pallor to the presence of anemia. J Gen Intern Med 1997; 12:102-6.
- Khusun H, Yip R, Schultink W, et al. World health organization hemoglobin cut-off points for the detection of anemia are valid for an indonesian population. J Nutr 1999; 129:1669-74.
- Stacy DL, Han P. Serum ferritin measurement and the degree of agreement using four techniques. Am J Clin Pathol 1992; 98:511-5.
- Shaw NS. Iron deficiency and anemia in school children and adolescents. J Formos Med Assoc 1996; 95:692-8.
- Anyinam C. Ecology and ethnomedicine: Exploring links between current environmental crisis and indigenous medical practices. Soc Sci Med 1995; 40:321-9.
- Patwardhan B, Warude D, Pushpangadan P, et al. Ayurveda and traditional Chinese medicine: A comparative overview. Evid Based Complement Altern Med 2005; 2:465-73.
- Fabricant DS, Farnsworth NR. The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 2001; 109:69-75.
- Henny J. Perbedaan efektifitas buah jambu biji merah dan buah belimbing manis terhadap kadar haemoglobin pada ibu hamil. J Keb 2014; 1-8.
- Rusdi NHP. Pengaruh pemberian jus jambu biji merah (Psidium Guajava L) terhadap kadar hemoglobin dan feritin serum penderita anemia remaja putri. Tesis Prog Magister Ilmu Bio Univ Andalas 2017; 1-11.
- Nur M. Pengertian anemia defisiensi besi. Tangerang: Medina Publishing 2008; 1-4.
- Steele S, Kroeun H, Karakochuk C. The effect of daily iron supplementation with 60 mg ferrous sulfate for 12 weeks on non-transferrin bound iron concentrations in women with a high prevalence of hemoglobinopathies. J Clin Med 2019; 8:180.
- WHO. Guideline: Daily iron supplementation in adult women and adolescent girls. Geneva, World Health Organization 2016.
- WHO. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva, World Health Organization 2016.
- Wood RJ, Ronnenberg AG. Modern Nutrition in Health and Disease. Baltimore, Md: Williams & Wilkins 2006; 193-222.
- Boggs DR. Fate of a ferrous sulfate prescription. Am J Med 1987; 82:124-8.
- Sahebzamani FM, Berarducci A, Murr MM. Malabsorption anemia and iron supplement induced constipation in post‐Roux‐en‐Y gastric bypass (RYGB) patients. J Am Assoc Nurse Practitioners 2013; 25:634-40.
- Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 2015; 126:1981-9.
- Ofer S, Fibach E, Kessel M, et al. Iron incorporation into ferritin and hemoglobin during differentiation of murine erythroleukemia cells. Blood 1981; 58:255-62.
- Hahn PF, Ross JF, Bale WF, et al. The utilization of iron and the rapidity of hemoglobin formation in anemia due to blood loss. J Exp Med 1940; 71:731-6.
- Hahn PF, Bale WF, Ross JF, et al. Radioactive iron absorption by gastro-intestinal tract: Influence of anemia, anoxia, and antecedent feeding distribution in growing dogs. J Exp Med 1943; 78:169-88.
- Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron- restricted erythropoiesis. Blood 2010; 116:4754-61.
- Daswani PG, Gholkar MS, Birdi TJ. Psidium guajava: A single plant for multiple health problems of rural Indian population. Pharmacogn Rev 2017; 11:167-74.
- Ojewole JA, Awe EO, Chiwororo WD. Antidiarrhoeal activity of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rodents. J Smooth Muscle Res 2008; 44:195-207.
- Rojas-Garbanzo C, Zimmermann BF, Schulze-Kaysers N, et al. Characterization of phenolic and other polar compounds in peel and flesh of pink guava (Psidium guajava L. cv. 'Criolla') by ultra-high performance liquid chromatography with diode array and mass spectrometric detection. Food Res Int 2017; 100:445-53.
Author Info
Mega1, Hidayat Wijayanegara1, Siti Sugih Hartiningsih1*, Menizibeya O Welcome2 and Senol Dane2
1Dharma Husada Institute of Health Sciences, Bandung, Indonesia2Department of Physiology, College of Health Sciences, Nile University of Nigeria, Abuja, Nigeria
Citation: Mega, Hidayat Wijayanegara, Siti Sugih Hartiningsih, Menizibeya O Welcome, Senol Dane, Effects of Red Guava Juice on Hemoglobin and Hematocrit Levels in Female Adolescent Students with Anemia, J Res Med Dent Sci, 2019, 7(3): 107-112.
Received: 01-May-2019 Accepted: 30-May-2019
