Research - (2020) Advances in Dental Surgery
Efficacy of Baclofen on Sequelae of Impacted Mandibular Third Molar Surgery
Swetha Bhat, Senthilnathan Periasamy* and Arun Murugaiyan
*Correspondence: Senthilnathan Periasamy, Department of Oral and Maxillofacial Surgery, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India, Email:
Abstract
Purpose: To evaluate the influence of the muscle relaxant Baclofen when used as a supplement to antibiotic and non-steroidal antiinflammatory treatment with the aim of reducing undesirable consequences after third-molar extraction such as trismus, pain and swelling. Materials and methods: This prospective clinical study was conducted on 64 patients who reported to the Department of Oral surgery, Saveetha Dental college, Chennai for the surgical removal of a mandibular impacted molar. These patients were randomly assigned to receive either Baclofen or the placebo. . The test group received Baclofen 5 mg once a day for the first 2 postoperative days, in addition to antibiotic and non-steroidal anti-inflammatory medications. Postoperatively, 1 independent investigator assessed the participant’s postoperative pain by means of a 10 point visual analog scale, swelling and maximal interincisor distance on the third and the seventh days. Results: In the test group, 21(65.62%) patients showed improvement of MMO above 8mm. The improvement in MMO was greater in the test group in comparison to the control group, but this was statistically insignificant. The mean of the VAS score for the test group on the 7th day was lesser than the VAS score of the placebo group, which showed better pain control and was statistically significant. Out of the patients who received Baclofen, 4 (12.5%) of them showed presence of swelling on the 7th postoperative day, whereas 7 (21.9%) patients in the control group presented with swelling. However, this was statistically insignificant. Conclusion: The results of this trial indicate that the influence of baclofen over pain, swelling and trismus does not rationalise prescribing additional medication for patients undergoing the surgical removal of a mandibular impacted third molar.
Keywords
Baclofen, Muscle relaxant, Third molars, Maximal mouth opening, Pain
Introduction
Teeth that fail to attain a functional position may be pathological and should be considered for removal which is common in oral surgical practice [1]. Surgical removal of impacted lower third molars is one of the most commonly performed surgical procedures in oral surgery [2]. Although it is a minor surgical procedure, it often involves pain, swelling, and dysfunction during the postoperative period [3–6]. The factors that contribute to these conditions are complex but lead to an inflammatory process initiated by surgical trauma [7]. It results in varying degrees of pain, swelling, and trismus [8,9]. Swelling and trismus usually reach their maximum level by the 1st or 2nd day postoperatively, begins to decrease on the 3rd or 4th day, and is usually resolved towards the end of the first week [10]. Pain reaches its maximum intensity 6-8 hours following surgery, continues for 2-3 days, and decreases by the seventh day [11,12].
It is necessary to identify causative factors and select treatment approaches accordingly to limit the clinical sequelae after the removal of third molars, reducing discomfort and increasing patient’s functional ability in the postoperative period [13]. Functional impairment and pain are considered as normal physiologic responses to operative injury [14]. Presence of any muscle spasm can be related to local factors involving affected muscle groups causing significant functional disability and pain [15,16]. Clinical studies have been done investigating methods to reduce postoperative sequelae such as the use of medications like analgesics, steroids, muscle relaxants, vitamins, and antibiotics, ice application, low dose laser therapy, different flap techniques, different closure techniques, drainage, and prp-prf procedures [7,17–24].
The muscle relaxants baclofen, dantrolene, and tizanidine are a heterogeneous group of medications approved for the treatment of spasticity [25]. Structurally, baclofen is related to the centrally occurring inhibitory neurotransmitter GABA. Clinically, it has commonly been used for its muscle relaxant effects in the treatment of spasticity, as well as for its neuropathic analgesic properties in the treatment of trigeminal neuralgia pain [26]. Baclofen is a GABA-B receptor agonist with presynaptic and postsynaptic effects leading to a decrease in the excitatory neurotransmitter release as well as in substance P, which is involved in transmission of nociceptive impulses [27]. Baclofen is rapidly absorbed after oral administration and its biotransformation is low [28]. With a rich case bank established over 3 decades we have been able to publish extensively in our domain [29–39]. Based on this inspiration we aim to determine the efficacy of baclofen in the relaxation of the muscles surrounding the surgical site after the removal of impacted mandibular third molars and, thereby, reducing pain, swelling and trismus.
Materials and Methods
Study set up
This prospective clinical study consisted of 64 patients who reported to the department of oral and maxillofacial surgery, Saveetha Dental College and Hospital, Chennai from June 2019 to March 2020 for the surgical removal of impacted mandibular third molar.
Preoperative
Factors such as angulation and difficulty index were assessed using orthopantomograph and periapical radiograph to ensure the similarity of the tooth inclinations on the basis of Winter’s classification [40] with only vertical and mesioangular positions. Comprehensive clinical examination of the surgical field to exclude any inflammatory symptoms such as mucosal swelling, hyperemia, or exudation was done. Data regarding the maximum mouth-opening ability as an index of trismus were obtained from patients as the maximum interincisal distance (MID, measured in mm) between the right upper and right lower central incisor by using a calibrated scale immediately before surgery.
Postoperative
The postoperative treatment protocol for all patients included prescription of 500 mg Amoxicillin every 8 hours and 500 mg paracetamol + 100mg aceclofenac (Zerodol P) every 12 hours for 3 days. In addition to the standard medication, the test group (32 patients) received 5 mg Baclofen orally on the night of the day of surgery and the night of the first postoperative day. Patients were required by protocol to return for follow-up on days 3 and 7 after surgery. The main variables of trismus and pain and the presence of edema were examined. The maximum mouth-opening ability was measured at both the postoperative appointments. Pain intensity was assessed on a 10-point visual analog scale (VAS) with point 0 indicating no pain and 10 indicating unbearable pain. Swelling was evaluated by measuring the distance between the angle of mandible to the tragus. Sutures were removed at the end of the trial on day 7.
Selection criteria
Inclusion criteria
Healthy patients with non-restorable mandibular third molar.
History of pain.
Removal of at least 1 lower bony impacted third molar with a difficulty index of 5 to 7 Pederson.
Only vertical and mesioangular positions were included.
Enrolment in the study was limited to patients of both genders aged 18 to 44 years.
Exclusion criteria
Patients younger than 18 years of age.
Past medical or drug history contraindicating the use of the study medication (e.g., allergy, severe health conditions, hypo- or hypertension, gastrointestinal disease, asthma).
Pregnancy.
Current signs and symptoms of acute infection or pain.
Use of antibiotics and/or analgesics within the 48-hour period before surgery.
Study parameters
Age of the patient.
Gender of the patient.
Angulation of the impacted tooth.
Preoperative and postoperative maximal mouth opening.
Postoperative VAS pain scores.
Preoperative and postoperative swelling.
Procedure
Surgical procedure was performed using local tissue infiltration and inferior alveolar nerve block (2% lignocaine with 200000 adrenaline). Standard Terence Ward’s incision or an envelope flap was raised in all cases and after reflecting the buccal flap, a gutter in the distobuccal bone was created to expose maximum contour of the tooth. Bone removal was done using a motor driven surgical bur under constant irrigation of normal saline. Odontectomy or odontomy procedure was performed depending on the path of removal of the impacted tooth [41]. Wound was carefully irrigated, and any bony spicules were removed, following which flap was repositioned and sutured using 2-0 silk. During the postoperative phase, all patients were given instructions about the wound and possible complications. All patients were prescribed the above-mentioned analgesics and antimicrobials as well as the muscle relaxant Baclofen to the test group, in the postoperative phase.
Data collection
The data related to the stay parameters were obtained from among the patients who reported tothe Department of Oral and Maxillofacial Surgery, Saveetha Dental College, Chennai from June 2019 to March 2020. An approval for the designed study was obtained from the Institutional Ethical Committee of Saveetha University (Ethical approval number SDC/SIHEC/2020/ DIASDATA/0619-0320). An informed verbal and written consent were obtained after explaining the nature of the procedure and the potential complications involved.
Data analysis
The IBM SPSS (version 23.0) software was used to tabulate and analyse the collected data. Nonparametric data was analysed using descriptive statistics measuring frequency and percentage. Pearson’s chi square test was used to assess the association between.
Results and Discussion
Demographic distribution
Out of the total 64 patients, 27 (42.2%) were females and 37 (57.8%) were males. Maximum number of patients belonged to the age group of 20-30 years with a mean of 25.78 ±6.5 years. 32 patients belonged to the test and control group each. Out of the total, 36 were mesioangular and the remaining 28 were vertical in angulation according to Winters classification.
Difficulty in mouth opening
Postoperative MMO between the means of both groups was 2.47, for postoperative MMO on the 3rd day was 2.13 and for post-operative 7th day was 1.75. However, these values did not differ significantly (P<0.05) (Table 1). Improvement in the MMO for the test group was calculated using the difference between preoperative and postoperative MMO on the 7th day. Figure 1 depicts that baclofen shows greater improvement in MMO in comparison to the control group. Chi square test was done to find the association between this improvement and gender. 15 (46.88%) males and 6 (18.75%) females showed improvement of MMO above 8mm. Males showed greater improvement in MMO by the 7th day in comparison to males (Figure 2). However, this was not statistically significant (P>0.05).
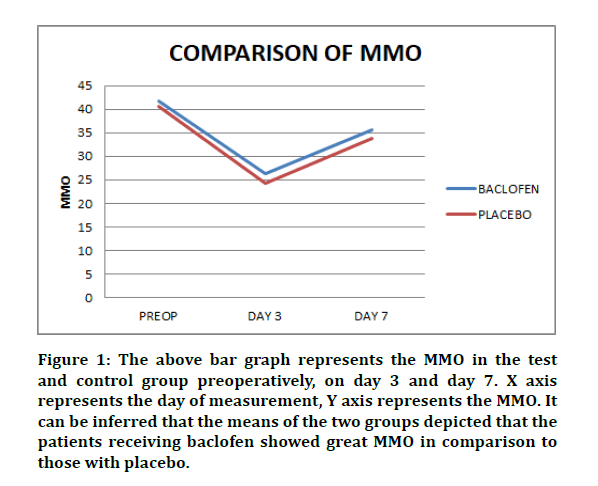
Figure 1: The above bar graph represents the MMO in the test and control group preoperatively, on day 3 and day 7. X axis represents the day of measurement, Y axis represents the MMO. It can be inferred that the means of the two groups depicted that the patients receiving baclofen showed great MMO in comparison to those with placebo.
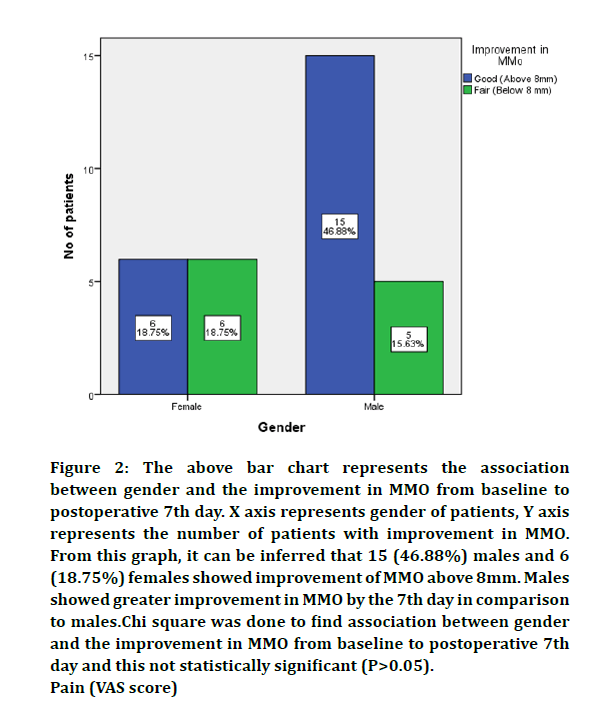
Figure 2: The above bar chart represents the association
between gender and the improvement in MMO from baseline to
postoperative 7th day. X axis represents gender of patients, Y axis
represents the number of patients with improvement in MMO.
From this graph, it can be inferred that 15 (46.88%) males and 6
(18.75%) females showed improvement of MMO above 8mm. Males
showed greater improvement in MMO by the 7th day in comparison
to males.Chi square was done to find association between gender
and the improvement in MMO from baseline to postoperative 7th
day and this not statistically significant (P>0.05).
Pain (VAS score)
| Baclofen | Placebo | Test value | P | |
|---|---|---|---|---|
| MMO- PREOP | 41.72 | 39.25 | 1.431 | 0.163 |
| MMO-3RD DAY | 26.41 | 24.28 | 1.391 | 0.174 |
| MMO-7TH DAY | 35.66 | 33.91 | 0.936 | 0.357 |
| VAS-3RD DAY | 5.09 | 7 | 8.43 | 0 |
| VAS-7TH DAY | 2.66 | 5.03 | 10.227 | 0 |
Table 1: This table represents the distribution of variables and comparative test results of the maximal mouth opening (MMO) preoperatively and postoperatively on day 3 and 7. The paired t-test between the test and control group was insignificant for MMO seen preoperatively and postoperatively on day 3 and 7. Whereas the paired t-test between the test and control group was significant for VAS scores on day 3 and 7.
The VAS values obtained for both treatment groups decreased significantly over time. At days 3 and 7, VAS values obtained from patients treated with Baclofen were slightly lower than those from patients who received no additional medication (Figure 3); with a difference of means in the test and control group being 1.91 on the 3rd day and 2.38 on the 7th day which was statistically significant (P<0.05) (Table 1).
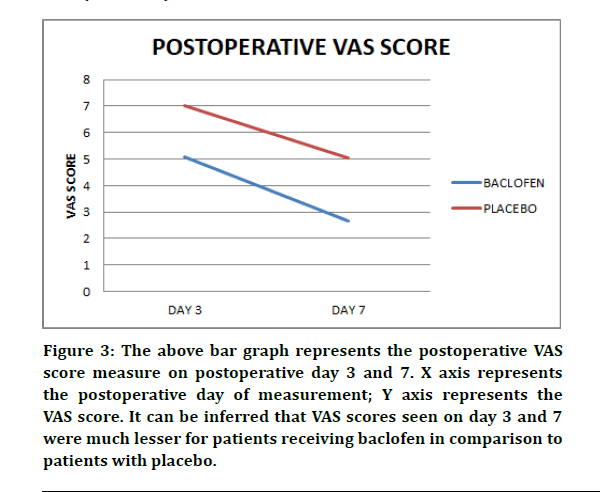
Figure 3: The above bar graph represents the postoperative VAS score measure on postoperative day 3 and 7. X axis represents the postoperative day of measurement; Y axis represents the VAS score. It can be inferred that VAS scores seen on day 3 and 7 were much lesser for patients receiving baclofen in comparison to patients with placebo.
Swelling
Out of the 32 patients who received Baclofen, 4 (12.5%) patients showed presence of swelling on the 7th postoperative day, whereas 7 (21.9%) patients in the control group presented with swelling (Figure 4).
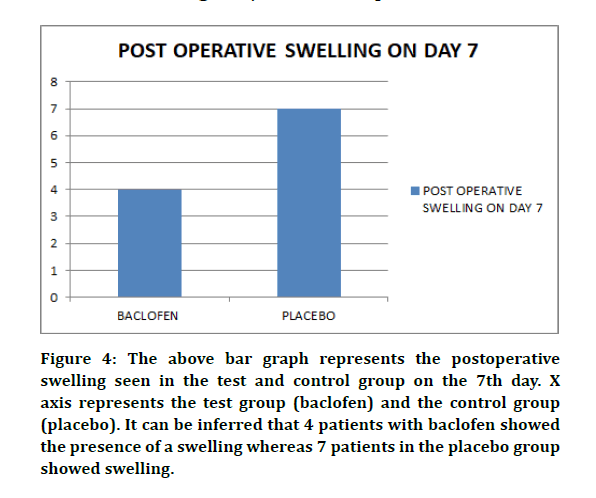
Figure 4: The above bar graph represents the postoperative swelling seen in the test and control group on the 7th day. X axis represents the test group (baclofen) and the control group (placebo). It can be inferred that 4 patients with baclofen showed the presence of a swelling whereas 7 patients in the placebo group showed swelling.
A number of investigative studies regarding the side effects that the patients experience after the surgical removal of impacted mandibular third molars have been published, but not many studies have been done on the influence of a muscle relaxant on postoperative swelling, pain, and decreased mouth opening. The purpose of our study was to obtain evidence regarding the efficacy of an oral dose of 5 mg of baclofen during the seven days after the surgical removal of an impacted mandibular third molar. We intended that the use of baclofen would supplement concomitant cryotherapeutic, antibiotic and non-steroidal anti-inflammatory treatment and, thus, reduce discomfort and increase the patient’s functional efficiency.
Oral baclofen is used more frequently than other antispasmodic agents to treat spasticity [42]. This drug was initially approved by the FDA in 1992 [43]. It has been observed that baclofen also has analgesic/antinociceptive actions as well [44]. Baclofen is mainly water soluble and so does not readily cross the blood-brain barrier [45]. Baclofen is rapidly absorbed after oral administration, and up to 80% of an oral dose is excreted in the urine, with only a limited hepatic metabolism [46]. Common side effects are weakness, sedation, and dizziness. At higher doses, baclofen can cause seizures, ataxia, and halluci- nations. Abrupt withdrawal should be avoided because it can precipitate seizures and hallucinations [26] . Given its structural similarity to tricyclic anti- depressants as well as potent anticholinergic properties, caution should be exercised when considering its use in the elderly or in patients with heart dis- ease. Likewise, concomitant use with monoamine oxidase inhibitors is absolutely contraindicated because this combination can cause a hyperpyretic crisis or even death [26]. There were no severe side effects, probably owing to the low dosage used.
Surgery results in diffuse accumulation of fluid in the interstitial space that can manifest itself internally as well as externally [13]. In our study, the values for swelling were very low and it was only assessed visually, it was not possible to clearly define any difference in the development of edema. When we compared the test group to control, baclofen did not influence the incidence of facial swelling that significantly after surgical removal of lower impacted third molar.
We also administered baclofen to study its influence on pain. Pain was evaluated on the basis of a 10-point VAS. However, assessment of pain using this scale is subjective. Compared with the control group, the oral administration of baclofen resulted in improved VAS scores during the postoperative period, and this was statistically significant.
Reports on limitation of maximal mouth opening after surgical removal of lower impacted third molar vary widely, from 14% to 59%. [47,48] Trismus is the simplest to measure and compare, as it can be done objectively [49].It is important to note that a possible reason for this demonstrable difference between the test and control groups in mouth-opening ability on days 3 and 7 could be because of the fact that the GABA-agonist baclofen is effective in the fields of anesthesia and pain management. In the present study, baclofen could have had only an antinociceptive effect increasing function in such a manner that mouth opening was improved at postoperatively. It has been pointed out that the ability of an agent to decrease pain sensitivity need not increase the patient’s real mouth opening ability but may instead enhance the patient’s capability and willingness to open maximally during measurements [50].
Improvement in the examined parameters during the first days after surgery has a fundamental influence on a patient’s quality of life during that period [51,52]. We found baclofen to be efficacious in decreasing postoperative mouth-opening limitation at days 3 and 7 after surgery, although the clinical implication is questionable, because there was only a slight improvement between the two groups. Because there was no significant difference in reduction of postoperative MMO and swelling between the 2 treatment groups, we reason that there is no meaningful benefit from adding baclofen to the standardized analgesic and anti-inflammatory medication to control postoperative side effects.
The limitations of our present study included the need for the patient’s perspective on the complications. Also, the duration or difficulty level of the surgery was not taken into consideration.
Conclusion
Reducing muscle spasm after third molar surgery would be a creditable goal,especially if the clinical healing were not compromised. The findings of the present study indicate that the postoperative use of baclofen did not provide an adequate difference in the mouth opening limitation or the swelling between the study and the control groups, but showed significant antinociceptive properties implying that it cannot be recommended as an additional medication after the surgical removal of a mandibular impacted molar.
Acknowledgement
The authors would like to acknowledge the help and support rendered by the department of oral and maxillofacial surgery and information technology of Saveetha Dental College and Hospitals for their constant assistance with the research.
Conflict of Interests
The authors declare no conflicts of interest.
References
- Balakrishnan G, Narendar R, Kavin T, et al.. Incidence of trismus in transalveolar extraction of lower third molar. J Pharm Bioallied Sci 2017; 9:S222–227.
- Ngeow WC, Lim D. Do corticosteroids still have a role in the management of third molar surgery? Adv Ther 2016; 33:1105–1139.
- Curran JB, Kennett S, Young AR. An assessment of the use of prophylactic antibiotics in third molar surgery. Int J Oral Surg 1974; 3:1–6.
- Bystedt H, Nord CE, Nordenram A. Effect of azidocillin, erythromycin, clindamycin and doxycycline on postoperative complications after surgical removal of impacted mandibular third molars. Int J Oral Surg 1980; 9:157–165.
- Pedersen A. Interrelation of complaints after removal of impacted mandibular third molars. Int J Oral Surg 1985; 14:241–244.
- MacGregor AJ, Hart P. Effect of bacteria and other factors on pain and swelling after removal of ectopic mandibular third molars. J Oral Surg 1969; 27:174–179.
- Capuzzi P, Montebugnoli L, Vaccaro MA. Extraction of impacted third molars. A longitudinal prospective study on factors that affect postoperative recovery. Oral Surg Oral Med Oral Pathol 1994; 77:341–343.
- Laureano Filho JR, Maurette PE, Allais M, et al. Clinical comparative study of the effectiveness of two dosages of dexamethasone to control postoperative swelling, trismus and pain after the surgical extraction of mandibular impacted third molars. Cephalalgia 2008; 54753:220.
- Trindade PAK, Giglio FPM, Colombini-Ishikiriama BL, et al. Comparison of oral versus sublingual piroxicam during postoperative pain management after lower third molar extraction. Int J Oral Maxillofac Surg 2011; 40:292–297.
- Hupp JR. Wound repair In: Peterson LJ, Ellise E, Hupp JR, Tucker MR. Contemporary Oral and Maxillofacial Surgery 4th Edn, St Louis: The CV Mosby Co 2003:54.
- Marković AB, Todorović L. Postoperative analgesia after lower third molar surgery: contribution of the use of long-acting local anesthetics, low-power laser, and diclofenac. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006; 102:e4–8.
- Lyons CJ, Bruce RA, Frederickson GC, et al. Age of patients and morbidity associated with mandibular third molar surgery. J Am Dent Association 1980; 101:240–245.
- Kirmeier R, Truschnegg A, Payer M, et al. Evaluation of a muscle relaxant on sequelae of third molar surgery: A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007; 104:e8–14.
- Mehlisch DR. The efficacy of combination analgesic therapy in relieving dental pain. J Am Dent Assoc 2002; 133:861–871.
- Redillas C, Solomon S. Prophylactic pharmacological treatment of chronic daily headache. Headache 2000; 40:83–102.
- Berry H, Hutchinson DR. A multicentre placebo-controlled study in general practice to evaluate the efficacy and safety of tizanidine in acute low-back pain. J Int Med Res 1988; 16:75–82.
- Seymour RA, Meechan JG, Blair GS. An investigation into post-operative pain after third molar surgery under local analgesia. Br J Oral Maxillofac Surg 1985; 23:410–418.
- Kaplan V, Eroğlu CN. Comparison of the effects of daily single-dose use of flurbiprofen, diclofenac sodium, and tenoxicam on postoperative pain, swelling, and trismus: A randomized double-blind study. J Oral Maxillofac Surg 2016; 74:1946.e1–1946.e6.
- Cigerim L, Eroglu CN. Comparison of clinical efficacies of preoperatively initiated naproxen sodium--Codeine phosphate in combination, diclofenac potassium, and benzydamine hydrochloride for pain, edema, and trismus after extraction of impacted lower third molar: A randomized double-blind study. J Oral Maxillofac Surg 2018; 76:495–502.
- Gopee P, Rikhotso E. Impacted mandibular third molars: The efficacy of prophylactic antibiotics and chlorhexidine mouthwash in preventing postoperative infections. S Afr Dent J 2017; 72:213–218.
- Kazancioglu HO, Kurklu E, Ezirganli S. Effects of ozone therapy on pain, swelling, and trismus following third molar surgery. Int J Oral Maxillofac Surg 2014; 43:644–648.
- Sekhar CH, Narayanan V, Baig MF. Role of antimicrobials in third molar surgery: prospective, double blind, randomized, placebo-controlled clinical study. Br J Oral Maxillofac Surg 2001; 39:134–137.
- Sortino F, Messina G, Pulvirenti G. Evaluation of postoperative mucosa and skin temperature after surgery for impacted third molar. Minerva Stomatol 2003; 52:393–399.
- Jackson DL, Moore PA, Hargreaves KM. Preoperative nonsteroidal anti-inflammatory medication for the prevention of postoperative dental pain. J Am Dent Assoc 1989; 119:641–647.
- Chou R, Peterson K, Helfand M. Comparative efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systematic review. J Pain Symptom Manage 2004; 28:140–175.
- Sharmila R. Muscle relaxants in treating tempromandibular joint disorder- An update. J Pharm Sci 2015; 7:611-614.
- Leibur E, Jagur O, Müürsepp P, et al. Long-term evaluation of arthroscopic surgery with lysis and lavage of temporomandibular joint disorders. J Craniomaxillofac Surg 2010; 38:615–620.
- Ghanavatian S, Derian A. Baclofen. Stat Pearls, Treasure Island (FL): Stat Pearls Publishing 2020.
- Senthil Kumar MS, Ramani P, Rajendran V, et al. Inflammatory pseudotumour of the maxillary sinus: clinicopathological report. Oral Surg 2019; 12:255–259.
- Wahab PUA, Madhulaxmi M, Senthilnathan P, et al. Scalpel versus diathermy in wound healing after mucosal incisions: A split-mouth study. J Oral Maxillofac Surg 2018; 76:1160–1164.
- J PC, Marimuthu T, Devadoss P, Kumar SM. Prevalence and measurement of anterior loop of the mandibular canal using CBCT: A cross sectional study. Clin Implant Dent Relat Res 2018; 20:531–534.
- Eapen BV, Baig MF, Avinash S. An Assessment of the incidence of prolonged postoperative bleeding after dental extraction among patients on uninterrupted low dose aspirin therapy and to evaluate the need to stop such medication prior to dental extractions. J Maxillofac Oral Surg 2017; 16:48–52.
- Marimuthu M, Andiappan M, Wahab A, et al. Canonical Wnt pathway gene expression and their clinical correlation in oral squamous cell carcinoma. Indian J Dent Res 2018; 29:291–297.
- Jain M, Nazar N. Comparative evaluation of the efficacy of intraligamentary and supraperiosteal injections in the extraction of maxillary teeth: a randomized controlled clinical trial. J Contemp Dent Pract 2018; 19:1117–1121.
- Abhinav RP, Selvarasu K, Maheswari GU, et al. The patterns and etiology of maxillofacial trauma in South India. Ann Maxillofac Surg 2019; 9:114–117.
- Sweta VR, Abhinav RP, Ramesh A. Role of virtual reality in pain perception of patients following the administration of local anesthesia. Ann Maxillofac Surg 2019; 9:110–113.
- Abdul Wahab PU, Senthil Nathan P, Madhulaxmi M, et al. Risk Factors for post-operative infection following single piece osteotomy. J Maxillofac Oral Surg 2017; 16:328–332.
- Ramadorai A, Ravi P, Narayanan V. Rhinocerebral mucormycosis: A prospective analysis of an effective treatment protocol. Ann Maxillofac Surg 2019; 9:192–196.
- Patil SB, Durairaj D, Suresh Kumar G, et al. Comparison of extended nasolabial flap versus buccal fat pad graft in the surgical management of oral submucous fibrosis: A prospective pilot study. J Maxillofac Oral Surg 2017; 16:312–321.
- Winter GB. Principles of exodontia as applied to the impacted third molar: A complete treatise on the operative technic with clinical diagnoses and radiographic interpretations. St Louis: American Medical Book 1926; 21–58.
- Lata J, Tiwari AK. Incidence of lingual nerve paraesthesia following mandibular third molar surgery. Natl J Maxillofac Surg 2011; 2:137–140.
- Rizzo MA, Hadjimichael OC, Preiningerova J, et al. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler 2004; 10:589–595.
- https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/021589s000_KemstroTOC.cfm#:~:text=Approval%20Date%3A%2010%2F30%2F2003
- Bowery NG. GABAB receptor: A site of therapeutic benefit. Curr Opin Pharmacol 2006; 6:37–43.
- Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. J Pharm Pharm Sci 2003; 6:252–273.
- Gerkin R, Curry SC, Vance MV, et al. First-order elimination kinetics following baclofen overdose. Ann Emerg Med 1986; 15:843–846.
- Beard S, Hunn A, Wight J. Treatments for spasticity and pain in multiple sclerosis: a systematic review. Health Technol Assess 2003;7: 1–111.
- Laureano Filho JR, de Oliveira e Silva ED, Batista CI, et al. The influence of cryotherapy on reduction of swelling, pain and trismus after third-molar extraction: A preliminary study. J Am Dent Assoc 2005; 136:774–778.
- Garcia Garcia A, Gude Sampedro F, Gandara Rey J, et al. Trismus and pain after removal of impacted lower third molars. J Oral Maxillofac Surg 1997; 55:1223–1226.
- Mehlisch DR. Evaluation of trismus, bite force, and pressure algometry after third molar surgery: A placebo-controlled study of ibuprofen. J Oral Maxillofac Surg 1998; 56:427–429.
- McGrath C, Comfort MB, Lo ECM, et al. Changes in life quality following third molar surgery – the immediate postoperative period. Br Dent J 2003; 194:265–268.
- Colorado-Bonnin M, Valmaseda-Castellón E, Berini-Aytés L, et al. Quality of life following lower third molar removal. Int J Oral Maxillofac Surg 2006; 35:343–347.
Author Info
Swetha Bhat, Senthilnathan Periasamy* and Arun Murugaiyan
Department of Oral and Maxillofacial Surgery, Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, IndiaCitation: Swetha Bhat, Senthilnathan Periasamy, Arun Murugaiyan, Efficacy of Baclofen on Sequelae of Impacted Mandibular Third Molar Surgery, J Res Med Dent Sci, 2020, 8 (7): 246-252.
Received: 16-Sep-2020 Accepted: 02-Nov-2020 Published: 09-Nov-2020
