Research - (2021) Volume 9, Issue 5
Efficacy of Smear Layer Removal from Root Canal Surface Using: Sonic, Ultrasonic, Different Lasers as Activation Methods of Irrigant (SEM study)
Huda S Al-baker* and Hussain F Al-Huwaizi
*Correspondence: Huda S Al-baker, Department of Conservative dentistry, collage of dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: This study aims to assess the Effectiveness of different irrigation activation techniques in removing smear layer from the confines of root canal using SEM analysis. Subjects/Materials and Methods: A total samples of 80 maxillary first molars with a single straight palatal root canal were prepared to size X4 (protaper Next, Dentsply) and assigned to 2 groups (n=40): normal saline and EDTA 17%, each group subdivided into five subgroups (n=8): conventional needle irrigation (CN), EndoActivator (EA), ultrasonic activated irrigation (UAI), Er.Cr.YSGG 2780nm laser, and Diode 940nm laser. Roots were split longitudinally then each half were divided into three regions corresponding to the apical, middle, and coronal. Scanning electron microscope investigations were accomplished. Three pictures for each region were taken and scored. Presence of smear layer was assessed using (5-grade scoring systems). Data were submitted for statistical analysis using nonparametric tests. Results: Activation of irrigants using Er.Cr.YSGG laser improved smear layer removal significantly (P < 0.05). sonic EndoActivator and ultrasonic show similar results. Lasing EDTA by 940nm Diode laser was more effective for removal of smear layer than normal saline at (P<0.05) and shows similar effect to Er.Cr.YSGG laser. Conclusion: laser activation of irrigation appears to have an interesting application in laser assisted endodontic therapy. Diode laser activation of EDTA provide a beneficial cost-effective choice for smear layer removal with added photothermal disinfection and opened the way for more studies to investigate the effect with different irrigation solution for the benefit of smear layer removal.
Keywords
Irrigation, Laser, Smear layer, Sonic, Ultrasonic
Introduction
The final successful outcome in treatment of root canal relies on complete cleaning and disinfection of the root canal, for the elimination or prevention of apical periodontitis, and termination of patient symptoms [1]. Therefore, mechanical instrumentation of root canal should always associate with canal irrigation, for debridement of those areas that cannot be reached by endodontic files, to eliminate debris and smear layer, and upgrade disinfection [2].
Air bubbles and vapor locks inside root canal prevent fluid movement inside the narrow complex areas of isthmuses, fins, and lateral canals. Therefore, the only successful way to clean these areas, is through improvement of the degree of contact of irrigant with the canal wall, by physical agitation using vibrational movements, ultrasonics, or pulsed lasers [3]. Manual irrigation with a positive pressure, is generally performed using a syringe attached to a side vented needle. While machine-operated irrigation techniques involve ultrasonic, sonic and recent systems of apical negative pressure (ANP) irrigation and laser [4].
Ultrasonically activated files induce streaming patterns which circulate irrigants and generate shear stresses that can be effective in irrigant activation thus smear layer removal [5], while sonically driven polymer tip vibration in a well-shaped and fluid-filled canal, results in a hydrodynamic phenomenon and intracanal waves [6].
The efficacy of 2,780 nm Er.Cr:YSGG laser, which is largely absorbable by water is due to real cavitation effects [7]. Accompanied by the shock waves [8] and along with secondary bubbles that enhance removal of smear layer from areas unreached by endodontic shaping instruments during canal preparation. It appears that laser activation of irrigant can also be achieved using near infrared diode lasers [9]. During laser irradiation of irrigant solutions with 940nm,980nm diode laser, the temperature of irrigation fluid inside root canal rises by up to 30 degrees Celsius [10]. This elevation in temperature improves the chemical reactions of alkaline irrigation solutions like EDTA [11]. This is of specific interest, as these are notably more compact and cheaper than solid-state erbium laser devices. This in vitro study aimed to evaluate and compare the effectiveness of different laser and non-laser-based irrigation activation techniques in smear layer removal from root canal dentine using normal saline and 17% EDTA irrigation solution.
Null hypothesis: There’s no difference in the efficiency of residual smear layer removal from the canal wall between different activation systems with normal saline irrigant solution as compared with EDTA irrigant solution.
Materials and Methods
Sample collection
Eighty human maxillary first molar teeth (freshly extracted) with patients age range (18 to 35 years-old) were collected for this study. The selection criteria include palatal root having a mature apex with a single straight canal, absence of resorption, decay, crack, fractures, or endodontic obturation. The presence of a single straight canal was recognized by dental radiograph and under dental operating microscope (Zeiss Pico Mora, Carl Zeiss, Obercochen, Germany).
Sample preparation
Decoronation of samples using a diamond disk (22x 0.4) (Komet Dental. Germany) was to gain a 14 mm standardized working length. A K-File #10 was inserted inside the canal, until the tip observed at the root apical end, then subtraction of 1mm from the measured length. samples were disinfected for 10 minutes using NaOCl 5.25% (Dentaflux, Madrid, Spain), and placed in saline solution (Vitulia 0.9% Laboratorios ERN S.A, Barcelona, Spain) at 6°C. All experimental working steps were carried out by single operator.
Root canal instrumentation
Root canal patency was obtained with a K-file#10 (Dentsply Maillefer, Ballaigues, Switzerland) and shaping to the working length was achieved using Protaper Next® rotary instruments (Dentsply Maillefer, Ballaigues, Switzerland) up to X4 (40/.06 size/taper) file and following the manufacturer’s instructions for use. Irrigation were performed with 1ml of 5.25% NaOCl between each instrumentation file during shaping procedure using a 31-gauge 2 side-vented needle (Ultradent,USA) held at 2 mm shorter than the working length.
Sample grouping
Samples were divided into two main groups (A, B) n=40 for each according to the final irrigation solution, as normal saline were used in group A, and EDTA 17% were used in group B, and then subdivided into five subgroups (1- 5) n=8 according to the activation system as follows
Subgroup A1, B1
Activation of irrigants using conventional needle irrigation (CN). Five ml of irrigant (normal saline for subgroup A1, EDTA 17% for subgroup B1) was placed inside the irrigator device (Oralcare, China) and its handpiece attached to 31-gauge double side vented needle.
During all irrigation phases, the needle was placed 2mm shorter than the determined working length.The needle moved 2-3mm up and down, and the flow rate of the device adjusted to be 0.3 ml\sec.
Subgroup A2, B2
Activation of irrigant using sonic driven Endoactivator. The medium-size polymer tip (25/.04) was used to clean the canals. The tip was fitted passively within the canal, 2mm shorter than the working length, and operated at 10,000 cpm for 60 seconds in three cycles 20min each with pumping action in short 2-3mm vertical strokes.
Subgroup A3, B3
Activation of irrigant using ultrasonic activated irrigation (UAI). S21 silver activator tip, size 25/0.02, length 21mm attached to ultra x ultrasonic activator (Ultra X, Eighteeth, China) in 3 cycles of ultrasonic activation for 20 seconds. The tip was held 1 mm from the working length in the centre of the canal and apical-coronal pumping movements 2-3 mm were made. So that 1 minute of ultrasonic activation was accomplished for each canal.
Subgroup A4, B4
Activation of irrigation using Er:Cr:YSGG laser. Agitation with Er:Cr:YSGG pulsed laser, (Biolase, Waterlase, Iplus, CA, USA) 2780 nm. Radial firing tip, A 200 μm diameter (Biolase Technology), 21 mm long fiber tip was utilised according to the manufacturer’s instructions: Calibration factor 0.85. Panel setting: Pave=1.25W, pulse energy 25 mj, pulse duration: 60 μs repetition rate: 50 Hz.
The laser fiber tip was inserted 2 mm short from the apex, and in contact mode, a helicoidal movement was performed from apical to coronal direction in a speed of 1mm/s, in three cycles, each cycle was achieved in 18 s resulting in a total irradiation time of 54 seconds according to manufacturer instructions and Al-Karadaghi et al. methodology [12].
Subgroup A5, B5
Activation of irrigation using Diode laser 940 nm. Specimens were irradiated by endodontic fiberoptic tip, (E2) with 200 μm tip diameter (Ezlase, epic x, Biolase, CA, USA), with 4 W panel setting, CW mode; the fiber tip inserted 2 mm from the apex, in contact mode, and helicoidal movement from apical to coronal direction in a speed of 1mm/s. This was accomplished in two cycles 18 seconds each. Resulting in a total laser activation time of 36 seconds as followed by manufacturer instructions and Al-Mafrachi et al. methodology [13].
A total of 5ml irrigation solution (normal saline for group A, 17% EDTA for group B) was used between cycles of activation for all subgroups and the final rinse of 5ml distilled water all in a flow rate of 0.3ml\sec. using irrigator device.
Root sectioning and preparation protocol for SEM
Sample sectioning were performed with diamond disks under 4X magnification dental loops. Two grooves were made on buccal and the lingual root surface until transparent root canal was visualized. Then, roots splitting was performed longitudinally using a single edge razor blade and a hammer into two halves.
The dehydration of specimens was made with ethyl alcohol using ascending concentrations of (30–100%). Each sample was evaluated for residual smear layer under a scanning electron microscope SEM (Tescan, Mira3, 2018 France). The samples were divided into three regions 4mm each represent apical, middle, and coronal then three points were selected from each region as follows:
Apical third: 2mm, 3mm, 4mm from the apex.
Middle third: 5mm, 6mm, 7mm from the apex.
Coronal third: 9mm, 10mm, 11mm from the apex.
The selected points were observed under 1000, 5000 x magnification. The images were scored according to the Hulsmann’s criteria which measured the presence, quantity and distribution of the smear layer [14].
The data were collected and analyzed statistically using the Statistical Package for the Social Sciences (SPSS Inc, Chicago, IL version 22).
The given data were qualitative in nature; therefore, nonparametric tests were used.
Weighted coefficient kappa (Kw) test was used to evaluate interobserver reproducibility. Wilcoxon sum rank test was used to measure the difference between final irrigation techniques. Kruskal-Wallis test was used for pairwise comparisons between activation techniques. Wilcoxon signed rank test was used for pairwise comparisons between root canal thirds. The significance level was set at (P # 0.05).
Results
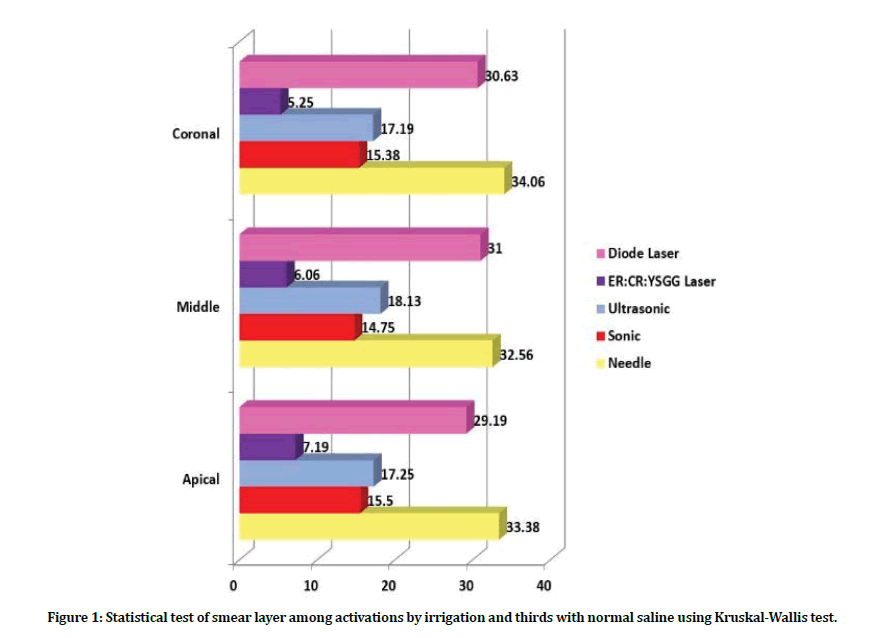
The inter examiner analysis exhibits good agreement (weighted kappa = 0.86) between both examiners. This indicate that both examiners were accurate. The Kruskal–Wallis analysis detected a significant difference between the different groups (p<0.05).
Intragroup comparison of smear layer: When comparing the amount of smear layer in group A (normal saline irrigation), subgroup A4 showed lower score of smear layer and with significant difference detected in apical third as compared with coronal third. While the highest score was detected in subgroups A5, A1 no matter what the third was. Regarding the results in subgroup A2, A3, the distribution of smear layer scores was comparable, with fluctuating manner of mean ranks from coronal to the apical third with no significant difference.
Regarding the different thirds: At the coronal third level: the least amount of smear layer regarding group A was found in subgroups A4, A2 with no significant difference between them. Followed by A3, A5, A1 subgroups respectively. At the middle third level: the least amount of smear layer was found in subgroup A4 followed by A2 and A3, respectively. The highest score level found in subgroup A5 and A1. At the apical third level: the least amount of smear layer was found in subgroup A4 followed by A2 and A3. While A5, A1 subgroups revealed the highest smear layer scores.
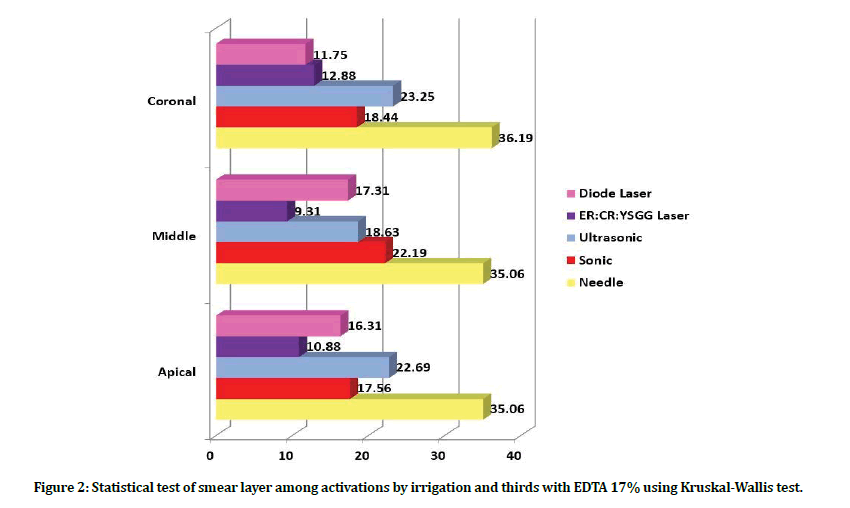
While the amount of smear layer presented in group B (when EDTA 17% solution used as irrigant) showed lower scores of smear layer in subgroup B4, B5 all over the coronal, middle and apical thirds. The results presented in subgroup B1 showed the highest smear layer at all thirds compared with B2, B3 subgroups that showed comparable results (Figure 1 and Figure 2).
Figure 1: Statistical test of smear layer among activations by irrigation and thirds with normal saline using Kruskal-Wallis test.
Figure 2: Statistical test of smear layer among activations by irrigation and thirds with EDTA 17% using Kruskal-Wallis test.
Regarding the different thirds: At the coronal third level: the least amount of smear layer found in subgroups B5 and B4 followed by subgroup B2 and B3, while the highest amount of smear layer was in subgroup B1. At the middle third level: the best smear layer removal was in subgroups B4, B5, B3 with no significant difference between them, followed by subgroup B2. The highest scores detected in subgroup B1 with significant difference with other subgroups. At the apical third level subgroups B4, B5 were the best in smear layer removal with no significant difference between them, followed by subgroups B2, B3 with no significant difference between them (Table 1 and Table 2).
Table 1: Multiple comparisons of smear layer in normal saline between activations by thirds using Wilcoxon sum rank test adjusted Dunn Bonferonni method.
| Thirds | Activation | ES | P value | Activation | ES | P value | ||
|---|---|---|---|---|---|---|---|---|
| Coronal | Needle | Sonic * | 0.804 | 0.001 | Sonic ^ | ER:CR:YSGG Laser | 0.4355 | 0.081 |
| Needle | Ultrasonic * | 0.726 | 0.004 | Sonic * | Diode Laser | 0.656 | 0.009 | |
| Needle | ER:CR:YSGG laser * | 1.2395 | 0.000 | Ultrasonic * | ER:CR:YSGG Laser | 0.5135 | 0.040 | |
| Needle | Diode Laser ^ | 0.148 | 0.554 | Ultrasonic * | Diode Laser | 0.578 | 0.021 | |
| Sonic | Ultrasonic ^ | 0.078 | 0.755 | Diode Laser* | ER:CR:YSGG Laser | 1.09175 | 0.000 | |
| Middle | Needle | Sonic * | 0.7675 | 0.002 | Sonic ^ | ER:CR:YSGG Laser | 0.3745 | 0.134 |
| Needle | Ultrasonic * | 0.62225 | 0.013 | Sonic * | Diode Laser | 0.70025 | 0.005 | |
| Needle | ER:CR:YSGG Laser * | 1.142 | 0.000 | Ultrasonic * | ER:CR:YSGG Laser | 0.51975 | 0.038 | |
| Needle | Diode Laser ^ | 0.06725 | 0.788 | Ultrasonic * | Diode Laser | 0.55475 | 0.026 | |
| Sonic | Ultrasonic ^ | 0.1455 | 0.561 | Diode Laser* | ER:CR:YSGG Laser | 1.07475 | 0.000 | |
| Apical | Needle | Sonic * | 0.769 | 0.002 | Sonic^ | ER:CR:YSGG Laser | 0.35775 | 0.153 |
| Needle | Ultrasonic * | 0.69375 | 0.006 | Sonic * | Diode Laser | 0.589 | 0.018 | |
| Needle | ER:CR:YSGG Laser * | 1.12675 | 0.000 | Ultrasonic ^ | ER:CR:YSGG Laser | 0.433 | 0.083 | |
| Needle | Diode Laser ^ | 0.18025 | 0.471 | Ultrasonic * | Diode Laser | 0.5135 | 0.040 | |
| Sonic | Ultrasonic ^ | 0.07525 | 0.763 | Diode Laser* | ER:CR:YSGG Laser | 0.9465 | 0.000 | |
Table 2: Multiple comparisons of smear layer in EDTA 17% between activations by thirds using Wilcoxon sum rank test adjusted Dunn Bonferonni method.
| Thirds | Activation | ES | P value | Activation | ES | P value | ||
|---|---|---|---|---|---|---|---|---|
| Coronal | Needle | Sonic * | 0.7925 | 0.002 | Sonic | ER:CR:YSGG Laser^ | 0.24825 | 0.321 |
| Needle | Ultrasonic * | 0.5775 | 0.021 | Sonic | Diode Laser ^ | 0.2985 | 0.232 | |
| Needle | ER:CR:YSGG Laser* | 1.04075 | 0.000 | Ultrasonic | ER:CR:YSGG Laser^ | 0.46325 | 0.064 | |
| Needle | Diode Laser * | 1.091 | 0.000 | Ultrasonic | Diode Laser * | 0.5135 | 0.040 | |
| Sonic | Ultrasonic ^ | 0.21475 | 0.390 | Diode Laser | ER:CR:YSGG Laser ^ | 0.05025 | 0.841 | |
| Middle | Needle | Sonic * | 0.569 | 0.023 | Sonic | ER:CR:YSGG Laser * | 0.569 | 0.023 |
| Needle | Ultrasonic * | 0.72625 | 0.004 | Sonic | Diode Laser ^ | 0.2155 | 0.389 | |
| Needle | ER:CR:YSGG Laser* | 1.13775 | 0.000 | Ultrasonic | ER:CR:YSGG Laser ^ | 0.4115 | 1.00 | |
| Needle | Diode Laser * | 0.78425 | 0.002 | Ultrasonic | Diode Laser ^ | 0.058 | 0.817 | |
| Sonic | Ultrasonic ^ | 0.1575 | 0.529 | Diode Laser | ER:CR:YSGG Laser^ | 0.3525 | 0.157 | |
| Apical | Needle | Sonic * | 0.75625 | 0.002 | Sonic | ER:CR:YSGG Laser^ | 0.289 | 0.248 |
| Needle | Ultrasonic * | 0.53475 | 0.032 | Sonic | Diode Laser^ | 0.054 | 0.829 | |
| Needle | ER:CR:YSGG Laser* | 1.045 | 0.000 | Ultrasonic | ER:CR:YSGG Laser* | 0.5105 | 0.041 | |
| Needle | Diode Laser * | 0.81025 | 0.001 | Ultrasonic | Diode Laser ^ | 0.2755 | 0.271 | |
| Sonic | Ultrasonic ^ | 0.2215 | 0.376 | Diode Laser | ER:CR:YSGG Laser ^ | 0.235 | 0.347 | |
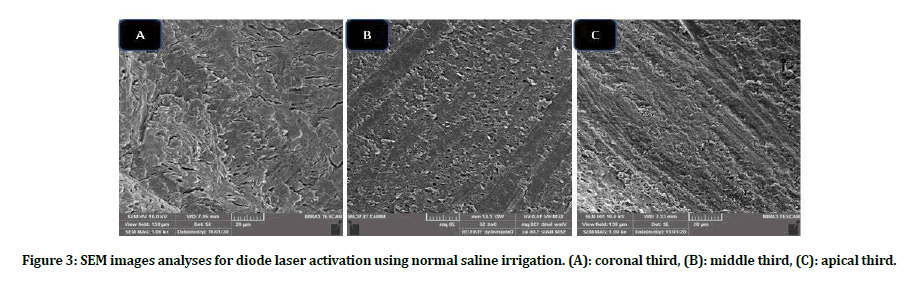
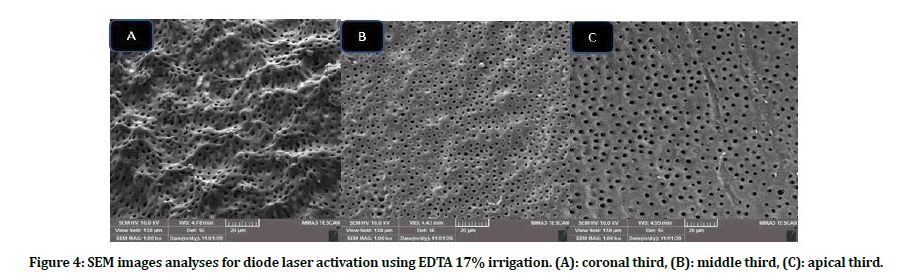
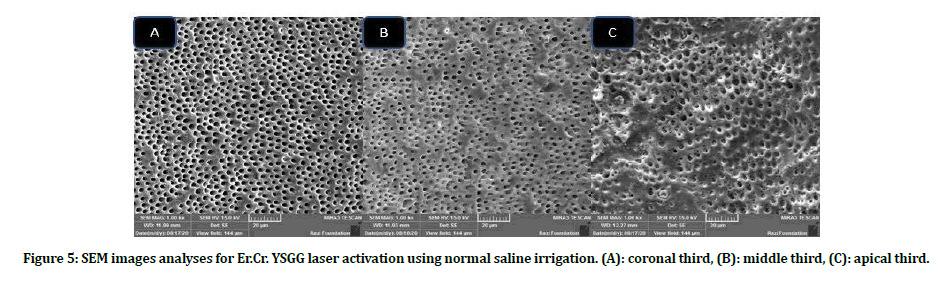
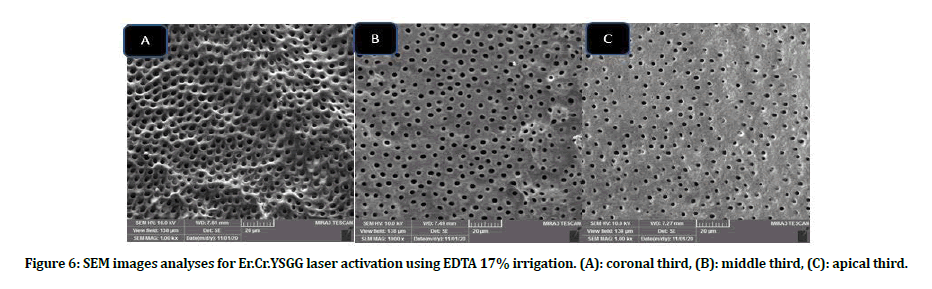
Intergroup comparison of smear layer: Group B scores presented better smear layer removal as compared with group A in all subgroups at all coronals, middle and apical thirds. Subgroups B4, B5 showed the lowest scores in term of smear layer removal, while A1, A5 subgroups revealed high smear layer scores at all locations when compared with other subgroups (Figures 3 to Figure 6).
Figure 3: SEM images analyses for diode laser activation using normal saline irrigation. (A): coronal third, (B): middle third, (C): apical third.
Figure 4: SEM images analyses for diode laser activation using EDTA 17% irrigation. (A): coronal third, (B): middle third, (C): apical third.
Figure 5: SEM images analyses for Er.Cr. YSGG laser activation using normal saline irrigation. (A): coronal third, (B): middle third, (C): apical third.
Figure 6: SEM images analyses for Er.Cr.YSGG laser activation using EDTA 17% irrigation. (A): coronal third, (B): middle third, (C): apical third.
Discussion
Smear layer generated during root canal instrumentation covers the root canal walls hindering the three-dimensional obturation and successful disinfection of the root canal system. Agitation of irrigation solutions is fundamental for further improvement of cleanliness of the root canal system. In the present study, the cleaning efficiency detected significant differences in smear layer removal between the activation groups, P<0.05.
Subgroup A1(CN with normal saline), B1(CN with EDTA) showed the presence of heavy smear layer throughout the length of the canals when conventional needle irrigation method was used with normal saline and with EDTA respectively, which is like a previous study by [15] which presented that needles or syringes used in conventional irrigation performed the least in debris and smear layer removal.
All activation techniques were significantly more effective than conventional needle except diode with normal saline (group A5) which might be due to the lack of interaction or absorption between diode laser and normal saline which is considered as inert solution, therefore no activation of irrigation or dissolution of smear layer [16].
Sonic agitation showed no significant difference to ultrasonic, which is like those reported by Urban et al who reported a similar effectiveness regarding removal of smear layer using #15 EndoActivator polymer tip and #20 ultrasonic tip and #40/0.02 apical sized canal [17]. The result in our study might be due to the limited space for ultrasonic tip in the root canal space to induce agitation of the irrigant fluid.
However, several studies indicating significant differences in the debridement efficacy between sonic and ultrasonic devices. Whereas ultrasonic agitation brought better removal of debris from root canal complex [18].
Regarding different thirds, mean rank varies between coronal and apical levels with significant differences were detected in A4 subgroup (Er. Cr.YSGG with normal saline) where the laser fiber tip makes more contact with the walls and a fused dentinal wall could be produced, and in B3 subgroup (Ultrasonic-EDTA), B5 ( Diode-EDTA), that’s might be due to limited space apically to oscillate and activate the EDTA solution, This finding is in consistent to the study of [19] in which removal of smear layer was significantly better in the coronal other than the apical third, independent of the type activation technique.
The findings in this study reported that the EndoActivator, ultrasonic system increase the efficiency of irrigation significantly better than diode laser with normal saline, which is attributed to the property of diode laser wavelength absorption with no affinity to normal saline. EndoActivator and ultrasonic vibration enhance the effect of EDTA by increasing the depth of penetration through dentinal tubules due to the acoustic streaming. However, they showed no difference with diode laser and EDTA at coronal, middle, and apical third [20].
Er.Cr.YSGG group showed more effective smear layer removal than other groups at all thirds similar to the finding of De Groot et al. [21] and this is suggested to be due to the shockwaves created by dental lasers within root canals that can play an essential role in removal of smear layer causing the initiation of vapor bubbles that expand during initiation of laser pulse and implode after termination of the pulse. The volumetric change in fluid associated with such an inertial collapse, added to the oscillations of smaller bubbles resulting in acoustic streaming, was thought to declare the efficacy of cleaning in m laser activated irrigation by Er.Cr.YSGG. The superior cleaning of the root canal wall was also allowed by secondary cavitational bubbles as they are excited by the bubble collapse of the consecutive laser pulse.
The parameters of diode laser utilized in this study based on the known threshold settings of 940 nm diode laser necessary to induce, cavitation, agitation and shockwaves [9]. The findings in our study illustrate that lasing EDTA solution using 940nm laser considerably improves smear layer removal. This result for the near-infrared laser aligns with previous research work which demonstrated improved removal of smear layer using mid-infrared erbium lasers [22].
It has been reported that the surface tension of the EDTA irrigant can be declined with an increase in temperature; as heating can cause expansion and a decrease in intermolecular attraction facilitates wetting of the root canal walls [23]. The debridement effect of both midinfrared lasers and near-infrared with EDTA could be attributed to temperature raising of EDTA solution along with physical fluid movement, which improves the cleaning efficacy through hydraulic stresses and shear forces activating the solution [24].
Thermal stress study by Al-Karadaghi et al. [25] proved that neither the erbium nor diode lasers when used in this parameters can cause changing in temperature, which would have unfavorable side effects on the health of the peri radicular tissues. On this basis, it is acceptable that the laser agitation protocol used in our study would be sound for clinical use.
Conclusion
Diode laser with 940 nm wavelength used to irradiate EDTA in the root canal system can significantly improve smear layer removal similar to Erbium laser, and this is preferred over sonic and ultrasonic by added photothermal disinfection property. Future studies should investigate the effects of Diode laser using different irrigation fluids to maximize the potential for smear layer removal. This study will open the way for most reliable cost-effective laser activation method compared with Erbium laser.
References
- Chubb DWRJAEJ. A review of the prognostic value of irrigation on root canal treatment success. 2019;45(1):5-11.
- Benetti CV, Panho N, Rafagnin GDJRBO. Strategies to optimize the removal of smear layer from the root canal system. 2018;75:e1314.
- Eggmann F, Vokac Y, Eick S, Neuhaus KWJBOH. Sonic irrigant activation for root canal disinfection: power modes matter! 2020;20:1-9.
- Gohil H, Mod DC, Shetty V, Kanaparthy A, Goswami HJJoAHS, Research, et al. Advances in activation of endodontic irrigants. 2020;1(1):52.
- Nusstein JM. Sonic and ultrasonic irrigation. Endodontic Irrigation: Springer; 2015. p. 173-97.
- Ruddle CJJDT. Endodontic triad for success: the role of minimally invasive technology. 2015;34(5):76-80.
- Saricam E, Küçük M, Akyol MJMR, Technique. Evaluation of EDTA, QMix, and Irritrol solutions activated with Er, Cr: YSGG and diode lasers on the push‐out bond strength of filling material. 2020.
- Betancourt P, Sierra JM, Camps-Font O, Arnabat-Domínguez J, Viñas MJA. Er, Cr: YSGG Laser-Activation Enhances Antimicrobial and Antibiofilm Action of Low Concentrations of Sodium Hypochlorite in Root Canals. 2019;8(4):232.
- Hmud R, Kahler WA, George R, Walsh LJJJoe. Cavitational effects in aqueous endodontic irrigants generated by near-infrared lasers. 2010;36(2):275-8.
- Hmud R, Kahler WA, Walsh LJJJoe. Temperature changes accompanying near infrared diode laser endodontic treatment of wet canals. 2010;36(5):908-11.
- Çiçek E, Keskin ÖJS. The effect of the temperature changes of EDTA and MTAD on the removal of the smear layer: a scanning electron microscopy study. 2015;37(3):193-6.
- Al‐Karadaghi TS, Al‐Saedi AA, Al‐Maliky MA, Mahmood ASJAEJ. The effect of bleaching gel and (940 nm and 980 nm) diode lasers photoactivation on intrapulpal temperature and teeth whitening efficiency. 2016;42(3):112-8.
- Al-Mafrachi R, Awazli L, Al-Maliky M, Ilps I. The Effect of using 940 nm Diode Laser in Comparison with Endoactivator on Radicular Dentin Permeability and Smear Layer Removal (An in Vitro Study). 2018:9-15.
- Hülsmann M, Rümmelin C, Schäfers FJJoE. Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. 1997;23(5):301-6.
- Orlowski NB, Schimdt TF, da Silveira Teixeira C, Garcia LdFR, Savaris JM, Tay FR, et al. Smear Layer Removal Using Passive Ultrasonic Irrigation and Different Concentrations of Sodium Hypochlorite. 2020;46(11):1738-44.
- Klim JD, Fox DB, Coluzzi DJ, Neckel CP, Swick MDJRW. The diode laser in dentistry. 2000;8(4):13-6.
- Urban K, Donnermeyer D, Schäfer E, Bürklein S. Canal cleanliness using different irrigation activation systems: a SEM evaluation. Clinical Oral Investigations. 2017;21(9):2681-7.
- Jiang L-M, Verhaagen B, Versluis M, van der Sluis LWJJoe. Evaluation of a sonic device designed to activate irrigant in the root canal. 2010;36(1):143-6.
- Haupt F, Meinel M, Gunawardana A, Hülsmann MJAEJ. Effectiveness of different activated irrigation techniques on debris and smear layer removal from curved root canals: a SEM evaluation. 2020;46(1):40-6.
- Abraham S, Vaswani SD, Najan HB, Mehta DL, Kamble AB, Chaudhari SDJJoCDJ. Scanning electron microscopic evaluation of smear layer removal at the apical third of root canals using diode laser, endoActivator, and ultrasonics with chitosan: An in vitro study. 2019;22(2):149.
- De Groot S, Verhaagen B, Versluis M, Wu MK, Wesselink P, Van Der Sluis LJIej. Laser‐activated irrigation within root canals: cleaning efficacy and flow visualization. 2009;42(12):1077-83.
- George R, Meyers IA, Walsh LJJJoE. Laser activation of endodontic irrigants with improved conical laser fiber tips for removing smear layer in the apical third of the root canal. 2008;34(12):1524-7.
- Ahmed S, Ismail PMS, Sekhar MC, Reddy SNL, Krishna MG, Reddy UN, et al. Evaluation of Effect of Irrigants with or without Surfactant on Root Canal Transportation by Cone Beam Computed Tomography–An In vitro Study. 2017;11(9):ZC75.
- Lagemann M, George R, Chai L, Walsh LJJAEJ. Activation of ethylenediaminetetraacetic acid by a 940 nm diode laser for enhanced removal of smear layer. 2014;40(2):72-5.
- Al-Karadaghi TS, Gutknecht N, Jawad HA, Vanweersch L, Franzen RJP, surgery l. Evaluation of temperature elevation during root canal treatment with dual wavelength laser: 2780 nm Er, Cr: YSGG and 940 nm diode. 2015;33(9):460-6.
Author Info
Huda S Al-baker* and Hussain F Al-Huwaizi
Department of Conservative dentistry, collage of dentistry, University of Baghdad, IraqReceived: 20-Apr-2021 Accepted: 06-May-2021 Published: 13-May-2021