Research - (2022) Volume 10, Issue 4
Evaluation of the Antibacterial Activity of Eucalyptus globules Essential Oil on Streptococcus mitis Bacteria
*Correspondence: Noora Maher Khudhiar, Department of Periodontology, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Resistance to synthetic antimicrobial agents has opened a new outlook in the search for natural alternatives. Plant-derived antibacterial compounds could be used in dentistry as a viable alternative treatment for oral disease. Among oral diseases, periodontal disease is caused by a variety of oral bacteria like Streptococcus mitis, the early colonizer of dental plaque. The study aims to evaluate the antibacterial effects of Eucalyptus globules essential oil against Streptococcus mitis, which is an early colonizer of dental plaque. Materials and Methods: Streptococcus mitis was isolated from a supragingival plaque sample of a subject. Streptococcus mitis sensitivity to various concentrations of Eucalyptus globules essential oil and compared with chlorhexidine mouthwash 0.2% as a +ve control and a distal water as a -ve control was determined by using the agar well diffusion. The Minimum Inhibitory Concentration (MIC) of essential oil against bacteria was determined using the broth microdilution method. The agar plate technique was used to find the Minimum Bactericidal Concentration (MBC) of the oil under test against the bacteria. Results: The Anti-Bacterial Activity (ABA) of Eucalyptus globules essential oil against Streptococcus mitis was shown to increase as the concentration of extract increased, with high significant differences (P ≤ 0.01) between all concentrations and chlorhexidine. Conclusion: The (ABA) of Eucalyptus globules essential oil against Streptococcus mitis suggests that it could be used as a natural antibacterial component in the treatment of oral infections.
Keywords
Eucalyptus globules, Essential oil, Streptococcus mitis, Dental plaque
Introduction
The oral cavity of a human being is mostly colonized by disease-causing microbes such as bacteria, protozoa, and viruses. Oral microorganisms are the reason of the most common oral human diseases: dental caries, periodontal diseases, and endodontic infection. As a result, plaquerelated diseases are likely to be the most frequent bacterial infections in people [1]. Dental plaque is a soft, non-mineralizing deposit that adheres tenaciously to the tooth surfaces, implants, and removable and fixed restorations. Plaque contains a diverse, highly organized aggregation of microorganisms that are embedded in an extracellular matrix of polymers [2]. Populations of microorganisms that are present in the plaque biofilm are involved in a series of metabolic, physical and molecular interactions that can modulate antibiotic resistance and pathogenicity. Antibiotic resistance in plaque biofilms is linked to a number of reasons, including the extracellular matrix's ability to act as the first defense line against antibiotic attack and facilitated gene transfer between microbes, among others. [3].These uncontrolled activities of pathogenic bacteria can result in health problems and the development of oral diseases. As a result, removing supra- and sub-gingival plaque biofilm is mostly beneficial for improvement of oral health [4]. Plaque control is done either chemically or mechanically, or both of them. Mechanical plaque control is carried out by tooth brushing and using interproximal cleaning aids such as interdental brushes and toothpicks [5].In spite of its crucial importance in the prevention of gingivitis and periodontitis, most individuals don't practice mechanical plaque control properly [6]. Studies have reported an increase in the prevalence of gingivitis and periodontitis that can be attributed to individuals’ reduction in the daily use of tooth brushing and interdental aids, as well as the accumulation of microbes on the soft oral tissues. This acts as a source of bacteria for colonization of the tooth surface. [7]. As a result, chemical plaque control is used in addition to mechanical plaque control to address mechanical plaque control's limitations and the large prevalence of gingivitis. [8].
Chemotherapeutic agents may inhibit or reverse gingivitis by decreasing dental plaque to a threshold value below which causes periodontal disease or altering the dental plaque bacterial composition such that health status may not turn into a disease. On the other hand, these agents can cause several side effects [9]. In order to avoid these risks, the antimicrobial activity of herbs and spices has been studied as an alternative to antibiotics. To date, the American Dental Association has approved just two medicines for the treatment of gingivitis: chlorhexidine digluconate mouthwash and essential oil mouth rinse [10]. Eucalyptus oil has important medicinally and pharmacologically influential chemicals that are utilized in several aspects of medicine as an antimicrobial, anti-inflammatory, antioxidative, antiseptic agent, antihistaminic, etc. [11]. An alternative hypothesis is that Eucalyptus essential oil shows an antibacterial effect against dental plaque primary colonizers.
Materials and Methods
Essential oil e preparation
Eucalyptus globules essential oil was purchased from Eden Garden .San Clemente.EXP.DATE.2022.
Sample collection
In this study, plaque sample was collected from a healthy person. By using sterilized Gracy curette, the sample was taken from supragingival plaque after isolating the tooth by a roll of cotton and dried by air flow to avoid contamination from saliva and other tissues. The sample was transferred immediately to 3 ml of Brain Heart Infusion Broth (BHIB) and immediately transported to the lab [12]. The exclusion criteria included: patients must not use antibiotic for at least four weeks before the study.
Isolation of Streptococcus mitis
The transported sample was well mixed by the vortex mixer and vibrated for 10–20 seconds after adding (5–6) small sterile glass beads (110–150 μm) into the small tube to improve sample dispersion [13,14]. Then some of this suspension was streaked by using a sterile inoculating loop, in duplicate on plates that have Mitis Salivarius Agar (MSA) media selective media for the isolation of Streptococci spp. Then, the plate was incubated for two days at 37°C anaerobically, followed by aerobic incubation at 37°C for one day.
Identifying Streptococcus mitis
Streptococcus mitis is identified based on conventional examinations: colony morphology on MSA media and blood agar media, Gram stain, biochemical tests, Optochin sensitivity test, and molecular identification by using conventional PCR techniques based on the specific 16S rRNA gene of Streptococcus mitis bacteria (Table 1).
| Primer Name | Sequences | Product size (bp) | References |
|---|---|---|---|
| S. mitis-F | ACAACTGAAACCTTTGCATCTGG | 391 | 14 |
| S. mitis-R | TCAAYTTCCAYGAYGCACCA |
Table 1: Sequences of Streptococcus mitis primer
In vitro experiments
Anti-bacterial activity (ABA)of various concentrations of Eucalyptus globules oil against Streptococcus mitis
The (ABA) of the extract was tested by using the agar well diffusion. The oil that was tested was made in four different concentrations: 100%, 75%, 50%, and 25% in dimethyl sulfoxide solution (DMSO). Four wells were pre-prepared in Mueller Hinton Agar (MHA) plates filled with 100μL of the different concentrations of the essential oil. Another Mueller Hinton Agar plate was prepared with two wells, one for chlorhexidine as a +ve control and one for distal water as a -ve control. Then the plates were incubated aerobically for 24 hours at 37◦C.
Determining the minimum inhibitory concentration (MIC) of the Eucalyptus oil on Streptococcus mitis
The (MIC) of Eucalyptus oil against Streptococcus mitis was determined using broth microdilution tests. For MICs, a variety of Eucalyptus oil concentrations were used, ranging from 25% to 0.09 percent v/v. As a brief overview, 100 μL of oil containing serial dilutions of the bacteria tested were placed in polystyrene sterile flatbottom 96-well plates. Using the DensiCHEK plus Meter, the initial bacterial inoculum was adjusted according to the McFarland standard for the test (suspension of bacteria containing 1.5 x 108 CFU/ml is equivalent to 0.5 McFarland). A well containing 0.2% chlorhexidine and a well containing bacterial inoculum without oil served as a positive and control, respectively. The plates were scanned with a spectrophotometer at 600 nm after being incubated aerobically for 24 hours at 37 °C. The minimum concentration of oil that did not demonstrate bacterial growth or turbidity after 24 hours of incubation in the broth microdilution method was calculated as an indicator of MIC. The experiments were carried out in triplicate.
Determining the minimum bactericidal concentration (MBC) of the Eucalyptus oil on Streptococcus mitis
The MBC is the minimum concentration of an antimicrobial agent necessary to kill 99.9% of the test organism in the original inoculum. MBC was determined by subculture the content of each well showed no bacterial growth on an agar plate and was incubated aerobically for 24 hours. MBC was the first with a higher or equal to MIC concentration with no growth [15].
Statistical analysis of the results
The data was analyzed using the Statistical Package For Social Science software version 25 (SPSS). The Analysis Of Variance test (ANOVA) was utilized to assess the inhibitory region between various concentrations of the oil being tested. Then, Tukey's test HSD (High Significant Differences) was employed to see if there was a statistically significant difference between the extract concentrations.
Results
Identification of Streptococcus mitis
Under the microscope, the selected colonies of Streptococcus mitis appeared as a gram +ve cocci. On Mitis salivarius agar medium, colonies appear as spherical or elliptical small, flat, and hard colonies, blue in color with a domed center, and the size of the colony is about 0.6– 0.8 μm in diameter. On blood agar plate, Streptococcus mitis forms small broken-glass-like colonies with alpha hemolysis. Streptococcus mitis tested catalase-negative, had a negative bile solubility test, and was resistant to the Optochin antibiotic test. On molecular identification of Streptococcus mitis, the results show amplification of the primer of bacterial species (391 size product and 51°C annealing temperature) which was fractionated on 1.5% agarose gel electrophoresis stained with Ethidium Bromide.
Antibacterial activity
The sensitivity of bacteria to different antimicrobial agents can be found by using the ager-diffusion method and microdilution tests for the determination of MIC and MBC of the antimicrobial agent.
Anti-bacterial activity of various concentrations of Eucalyptus globules Essential oil against Streptococcus mitis bacteria in comparison with chlorhexidine (CHX) mouthwash by ager diffusion method
The agar well diffusion techique was used to test the sensitivity of Streptococcus mitis to various concentrations of Eucalyptus globules oil, distal water (D.W), and CHX mouthwash 0.2% in vitro. Streptococcus mitis was sensitive to all essential oil concentrations. Antibacterial activity of Eucalyptus globules essential oil against Streptococcus mitis was discovered, and growth inhibition regions were created (clean regions around the wells without bacterial growth). An inhibition zone of greater than 8 mm indicates the sensitivity of the tested bacteria to the Eucalyptus oil [16]. The antibacterial activity was shown to increase as Eucalyptus oil concentrations was increased. 100% concentration showed the larger inhibition zone while the D.W (negative control) showed no inhibition zone.
Data distribution.
First, Shapiro-Wilk test was made to test whether the data of all concentrations of Eucalyptus oil and CHX were normally distributed or not (Table 2). It is shown by the Shapiro-Wilk test that data are normally distributed in the different concentrations of Eucalyptus oil and CHX because all p values were greater than the 0.05 level of significance (p>0.05), which allows for the use of conventional statistical methods, such as descriptive statistics expressed by mean and standard deviation, or inferential statistical methods such as parametric hypothesis.
| Concentrations % | Shapiro â??wilk | D.F. | p-value |
|---|---|---|---|
| 100% | 0.95 | 10 | 0.673 |
| 75% | 0.927 | 10 | 0.419 |
| 50% | 0.958 | 10 | 0.768 |
| 25% | 0.938 | 10 | 0.526 |
| 0.2%CHX | 0.82 | 10 | 0.251 |
Table 2: Shapiro-wilk test for normality distribution of data of all concentrations
A one-way Analysis of Variance (ANOVA) statistical test was used to perform a comparison among various concentrations of Eucalyptus oil and control agents. The High significant differences (P ≤ 0.01) were found among various concentrations of oil and control agents, as shown in Table 3 and Figure 1.
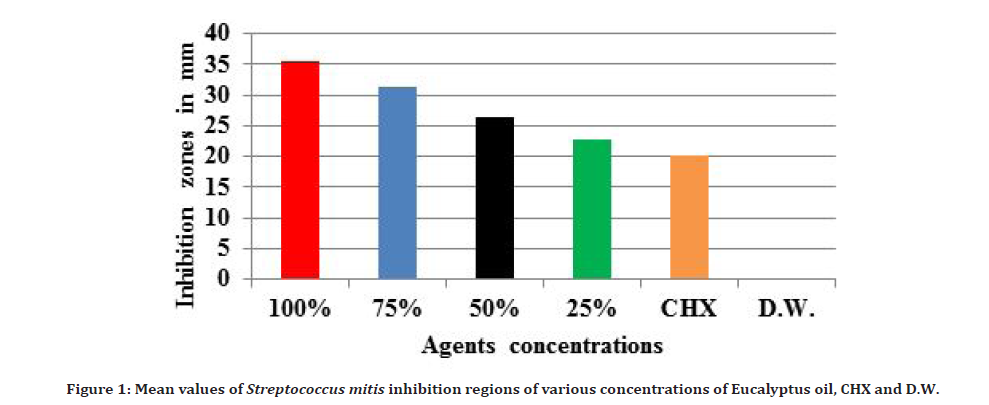
Figure 1. Mean values of Streptococcus mitis inhibition regions of various concentrations of Eucalyptus oil, CHX and D.W.
| Con. % | Descriptive statistics | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| NO. | Mean | S.D. | Mini. | Maxi. | F-test | P-values | |
| 100% | 10 | 35.4 | 3.239 | 31 | 41 | 291.034 | 0 |
| 75% | 10 | 31.3 | 3.02 | 27 | 36 | ||
| 50% | 10 | 26.5 | 2.369 | 22 | 30 | ||
| 25% | 10 | 22.8 | 2.44 | 18 | 26 | ||
| 0.2%CHX | 10 | 20.2 | 0.789 | 19 | 21 | ||
| D.W. | 10 | 0 | 0 | 0 | 0 | ||
Table 3: The statistical analysis of Streptococcus mitis inhibition regions by various concentrations of Eucalyptus oil, CHX and D.W .
Because the differences between various concentrations of Eucalyptus oil and the control agents were highly significant, a statistical comparison between each pair of various concentrations of the oil and the control agents was performed using Tukey's test HSD, as shown in Table 4. The results indicated high significant differences (P ≤ 0.01) between all concentrations of Eucalyptus oil and CHX, except between 25% and CHX, where the difference was not significant (P>0.05).
| Concentration % | Mean differences | Â P-value | Description | |
|---|---|---|---|---|
| 100% | 75% | 4.1 | 0.003 | H.S |
| 50% | 8.9 | 0 | H.S | |
| 25% | 12.6 | 0 | H.S | |
| CHX | 15.2 | 0 | H.S | |
| D.W. | 35.4 | 0 | H.S | |
| 75% | 50% | 4.8 | 0 | H.S |
| 25% | 8.5 | 0 | H.S | |
| CHX | 11.1 | 0 | H.S | |
| D.W | 31.3 | 0 | H.S | |
| 50% | 25% | 3.7 | 0.009 | H.S |
| CHX | 6.3 | 0 | H.S | |
| D.W | 26.5 | 0 | H.S | |
| 25% | CHX | 2.6 | 0.135 | N.S |
| D.W | 22.8 | 0 | H.S | |
| D.W | 20.2 | 0 | H.S | |
Table 4: Tukey's test for pair of concentrations of Eucalyptus oil, CHX and D.W against Streptococcus mitis
Determination of minimum inhibitory (MIC) and minimum bactericidal concentrations (MBC) of Eucalyptus essential oil against Streptococcus miti
The MIC and MBC of Eucalyptus oil were found against Streptococcus mitis, and the result showed that Eucalyptus oil had bacteriostatic and bactericidal activity on Streptococcus mitis. The MIC and MBC were both 1.5% v\v.
Discussion
In dentistry, many naturally derived and many synthetically derived antimicrobials were used to inhibit plaque biofilm formation. These are chlorhexidine gluconate and other antimicrobial agents such as minocycline, metronidazole, and doxycycline. These products have numerous uses, such as mouthwash, intra-pocket local drug delivery agents or topically applied gels. These modes of treatment have shown promise as adjuncts to conventional therapy scaling and root planning [17,18]. Dental communities are on the search for newer therapeutic agents that, in addition to improving periodontal health, do not have the usual antimicrobial adverse effects. Essential oils, for example, include phytochemicals that can be used as an alternative [19].
In this study, Eucalyptus Globulus essential oil was found to be antibacterial against Streptococcus mitis. This result shows an agreement with study [20] where the essential oil of Eucalyptus showed anti-bacterial activity against gram+ve bacteria. In agreement with the results given in detailed documentation on essential oils' antimicrobial properties [21], the sensitivity of Streptococcus mitis to Eucalyptus oil was found to increase with increasing concentration. The concentrations tested of Eucalyptus oil had stronger antibacterial activity than 0.2% chlorhexidine mouthwash in the agar diffusion method, and statistical analysis revealed high significant differences (P ≤ 0.01) between them. Eucalyptus oil inhibited the growth of Streptococcus mitis, and the minimum inhibitory concentration was 1.5%. This result coincides with that of [22], where the essential oil of Eucalyptus oil gave anti-bacterial activity against oral bacteria. Many studies have found that the antibacterial activity of volatile compounds such as Eucalyptus oil is due to a combination of indirect absorption by the medium that absorbed the vapor and direct vapor absorption by microbes [23].
Other studies have linked the antibacterial properties of Eucalyptus essential oil to active biological components such as oxygenated monoterpenes, monoterpenes, and oxygenated sesquiterpenes. Eucalyptus leaf essential oil contains higher than 70% (v/v) 1, 8-cineole [24]. Specifically, these compounds affect fatty acids in the bacterial cell membrane and cytoplasm, as well as proteins, ATP, cell morphology, and anti-quorum sensing activities [25].
Conclusion
The present work demonstrated the antibacterial efficacy of Eucalyptus globules essential oil against Streptococcus mitis, which may pave the way to preventing the development of biofilm formation by Streptococcus mitis and other primary plaque colonizers and improving oral and periodontal health.
References
- Chinsembu KC. Plants and other natural products used in the management of oral infections and improvement of oral health. Acta Tropica 2016; 154:6-18.
- Valm AM. The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. J Mol Biol 2019; 431:2957-2969.
- Digel I, Kern I, Geenen EM, et al. Dental plaque removal by ultrasonic toothbrushes. Dent J 2020; 8:28.
- Schmidt JC, Zaugg C, Weiger R, et al. Brushing without brushing?â??a review of the efficacy of powered toothbrushes in noncontact biofilm removal. Clin Oral Investigat 2013; 17:687-709.
- Graves RC, Disney JA, Stamm JW. Comparative effectiveness of flossing and brushing in reducing interproximal bleeding. J Periodontol 1989; 60:243-247.
- Teles FR, Teles RP, Uzel NG, et al. Early microbial succession in redeveloping dental biofilms in periodontal health and disease. J Periodont Res 2012; 47:95-104.
- Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2005; 38:135-87.
- Sugano N. Biological plaque control: Novel therapeutic approach to periodontal disease. J Oral Sci 2012; 54:1-5.
- Mhaske M, Samad BN, Jawade R, et al. Chemical agents in control of dental plaque in dentistry: An overview of current knowledge and future challenges. Adv Appl Sci Res 2012; 3:268-272.
- Shantipriya R. Essentials of clinical periodontology and periodontics. 2nd Ed. Jaypee Brothers Publishers 2008.
- Alashbal LA, Jafar ZJ, Aldhaher ZA. Antimicrobial Effect of eucalyptus oil as a root canal filling material for primary teeth in comparison with other filling materials against C. albicans and Streptococcus spp. J Pure Applied Microbiol 2019; 13:1537-43.
- Hohwy J, Reinholdt J, Kilian M. Population dynamics of Streptococcus mitis in its natural habitat. Infect Immun 2001; 69:6055-6063.
- Zhou X, Li Y. Atlas of oral microbiology: From healthy microflora to disease. 2020.
- Banas JA, Zhu M, Dawson DV, et al. PCR-based identification of oral streptococcal species. Int J Dent 2016; 2016.
- Thosar N, Basak S, Bahadure RN, et al. Antimicrobial efficacy of five essential oils against oral pathogens: An in vitro study. Eur J Dent 2013; 7:S071-7.
- Saquib SA, AlQahtani NA, Ahmad I, et al. Evaluation and comparison of antibacterial efficacy of herbal extracts in combination with antibiotics on periodontal pathobionts: An in vitro microbiological study. Antibiotics 2019; 8:89.
- Elias-Boneta AR, Toro MJ, Noboa J, et al. Efficacy of CPC and essential oils mouthwashes compared to a negative control mouthwash in controlling established dental plaque and gingivitis: A 6-week, randomized clinical trial. Am J Dent 2015; 28:21A-6A.
- Matesanz-Pérez P, GarcÃa-Gargallo M, Figuero E, et al. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J Clin Periodontol 2013; 40:227-241.
- Hans VM, Grover HS, Deswal H, et al. Antimicrobial efficacy of various essential oils at varying concentrations against periopathogen Porphyromonas gingivalis. J Clin Diagnost Res 2016; 10:ZC16.
- Bahjat SA. Evaluation of antibacterial and antibiofilm activity of cinnamon, clove, eucalyptus, and tea tree oils against oral Streptococci. Rafidain J Sci 2019; 28:1-4.
- Chouhan S, Sharma K, Guleria S. Antimicrobial activity of some essential oilsâ??present status and future perspectives. Med 2017; 4:58.
- Takarada K, Kimizuka R, Takahashi N, et al. A comparison of the antibacterial efficacies of essential oils against oral pathogens. Oral Microbiol Immunol 2004; 19:61-64.
- Trivedi NA, Hotchandani SC. A study of the antimicrobial activity of oil of Eucalyptus. Indian J Pharmacol 2004; 36:93.
- Brooker MIH, Kleinig DA. Field guide to eucalyptus. South-eastern Australia, 3rd Edn. Bloomings, Melbourne, Australia 2006.
- Nazzaro F, Fratianni F, De Martino L, et al. Effect of essential oils on pathogenic bacteria. Pharma 2013; 6:1451-74.
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar,, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Author Info
1Department of Periodontology, College of Dentistry, University of Baghdad, IraqReceived: 27-Mar-2022, Manuscript No. JRMDS-22-58668; , Pre QC No. JRMDS-22-58668 (PQ); Editor assigned: 29-Mar-2022, Pre QC No. JRMDS-22-58668 (PQ); Reviewed: 12-Apr-2022, QC No. JRMDS-22-58668; Revised: 15-Apr-2022, Manuscript No. JRMDS-22-58668 (R); Published: 22-Apr-2022
