Research - (2022) Volume 10, Issue 1
Evaluation of the Anti-Bacterial Effect of Ginger Aqueous Extract on Bacterial Flora of Patient with Periimplantitis
Ahmed Ghaith Ahmed1 and Abdalbseet A Fatalla2*
*Correspondence: Abdalbseet A Fatalla, Department of Prosthodontics, College of dentistry, University of Baghdad, Iraq, Email:
Abstract
Aim: To determine the concentration of aqueous ginger extract that inhibits the growth of anaerobic bacterial flora of patient with periimplantitis. Material and Methods: It included 12 partially edentulous subjects (12 males) aged 40-65 years with one or more periimplantitis implants. Peri-implantitis criteria, probing depth was 4-6 mm, bleeding on probing at one or more site, radiographic bone loss was about 0.5-2 mm. Subgingival bacterial specimens were collected from infected implants of each person using sterile curate and immediately transported through anaerobic transport media. Results: Revealed that aqueous ginger extract minimum inhibition concentration for Total anaerobic bacteria was (25% percent) on blood agar media. Conclusion: The antibacterial activity increased from 25% percent and above with the rise in the concentration of aqueous ginger extract, and selenium nanoparticles had no impact at 0.5 percent, 1 percent, 2 percent concentration.
Keywords
Minimum inhibition concentration, Aqueous ginger extract, Anaerobic bacteria
Introduction
Drug resistance is increasing worldwide and it is consider as a main culprit in the failure of treatment. The use of antibiotics against bacteria/microorganism is effective mode of treatment but also causes adverse complications. Earlier investigators have shown that, ginger and its constituents play a vital role in the prevention of microbial growth or acts as antimicrobial agents. An important study in the favors of ginger as anti-microbial activity showed that ginger has antimicrobial activity against E coli, Salmonella typhi and Bacillus subtilis and ethanolic extract of ginger showed widest zone of inhibition against Salmonella typhi [1]. Ginger rhizome contains several constituents which have antibacterial and anti-fungal effects. The gingerol and shagelol are identified as more active agents [2]. Earlier studies have shown that, ginger has broad antibacterial activity and the ethanolic extract of ginger powder has pronounced inhibitory activities against Candida albicans [3-5] and other report also showed that antifungal properties of ginger extract, Gingerol [6]. Chief constituents such as [6]-gingerol and [12]-gingerol, isolated from ginger rhizome, showed antibacterial activity against periodontal bacteria [6-8] and [10]-gingerol has been reported as active inhibitor of M. avium and M. tuberculosis in vitro [8].
Zingiber officinale (ginger), a horizontal, branched, fleshy, aromatic white to yellow coloured perennial herb with leafy stem up to 60 cm, has long been used in the field of medicine. It belongs to the family Zingerberaceae. Its leaves are narrow being 20 cm long and 1.5-2cm wide. It has a dense spiked, yellow green with purple ending flowers are seen [9]. 1- The rhizome is rich in secondary metabolites such as phenolic compounds (gingerol, paradol and shogaoal), volatile sesquiterpenes (zingiberene and bisabolene) andmonoterpenoids (curcumene and citral) Ginger has been widely used all over the world in ayurvedic medicine, for a wide array of unrelated ailments including arthritis, cramps, rheumatism, sprains, sorethroats, muscular aches, pains, constipation, vomiting, hypertension, indigestion, dementia, fever and infectious diseases [10]. 2-It has direct anti-microbial activity and thus can be used in treatment of bacterial infections [11]. 3-Ginger are relatively inexpensive due to their easy availability, universally acceptable and well tolerated by the most people. It has also been “Generally Recognized as Safe” (GRAS) by the US FDA [12].
Peri-implantitis has been associated with a gramnegative anaerobic microbiota, similar to that found in severe periodontitis around natural teeth. Periimplantitis encompasses the criteria of peri-mucositis and the addition of loss of osseous support. Although some natural bone remodeling post implant placement is normal, a stabilized implant that continues to exhibit bone level change is indicative of peri-implantitis.
Although bacterial insult is identified as the main cause of peri-mucositis, peri-implantitis is considered to be initiated by stress factors caused by a poor biomechanical environment. In addition, several other factors exist, such as poor implant placement, poor oral hygiene, residual cement, the body's rejection, poor implant surface, unfavorable osseous density, untreated periodontitis, drinking and smoking, untreated endodontic lesions, diabetes, etc. More etiologies are being identified as studies continue. Unfavorable stress factors can initiate crestal bone loss, and bacterial presence can further propagate the rate of osseous destruction. In recent studies it was found that bacterial biofilms attached onto the surface of implants can create a highly acidic environment that causes corrosion, pitting, cracking, etc. Furthermore, new studies have shed light on the release of titanium ions from the implant surface, which causes a significant increase in local inflammatory effect [13].
Materials and Methods
Ginger extracts preparation
Plant materials
Ginger (Zingiber officinale) was purchased from local market of medical herbs (Assuit). The plants were brought to the laboratory and thoroughly washed in distilled water and dried in shade at room temperature then stored in a plastic zip bag in at 4 ºC until use.
Powdered ginger extract preparation
Plant materials were finely grinded to powder by using a blender. One hundred gram of ginger mixed with one liter of sterile deionized water and kept in a water bath at 60ºC for five hours, then filtered through sterile filter paper “Whatman, UK”. The filtrates were exposed at 40 ºC to a hot air oven for evaporation of water. The filtrates were kept at 4 ºC until use [14].
Preparation of transport media (thioglycolate broth)
The preparation of thioglycolate media is done by
• Mixing powder of media with deionized water according to manufacturer instruction.
• Stir the media with magnetic stirrer and heater is set at 50 C° for 15 minutes until all the media has been dissolved completely.
• The next step is too sterile the media with autoclave to prevent contamination (Figure 1).
Figure 1. Preparation of transport media (thioglycolate broth).
The test bacterial isolates
The 1st test isolate from patient with periimplantitis attends to surgical department at collage of dentistry\ university of Baghdad for total flora account ,the sample is taken by using sterile curette inserted into the pocket depth and collect subgingival plaque ,after that immediately transported into transport media (thioglycolate broth). After that the broth incubated under anaerobic condition by using anaerobic jar and anaerobic gaz pack for 24 hours at 37 °C for bacterial activation and growth to prepare a bacterial source for later on culturing on agar media to achieve Kirby Bauer Disc Diffusion for ginger extract susceptibility test.
Kirby bauer disc diffusion method for antibiotic susceptibility testing [15]
Preparation of blood agar plates (culture media)
Composition of blood agar
0.5% Peptone, 0.3% beef extract/yeast extract, 1.5% agar, 0.5% NaCl, Distilled water, (Since Blood Agar is made from Nutrient Agar, above is the composition of Nutrient Agar), 5% Sheep Blood, the pH should be from 7.2 to 7.6 (7.4).
Preparation of blood agar
1-Suspend 28 g of nutrient agar powder in 1 liter of distilled water, then Heat this mixture while stirring to fully dissolve all components. Autoclave the dissolved and was mixture at 121 degrees Celsius for 15 minutes. Once the nutrient agar has been autoclaved, allow it to cool but not solidify. When the agar has cooled to 45-50 °C, Add 5% (vol/vol) sterile defibrinated blood that has been warmed to room temperature and mix gently but well by Swirl the flask to mix thoroughly. Avoid Air bubbles. Dispense into sterile plates while liquid, continuing to avoid bubbles and froth on the surface. (NOTE: Cooling the agar and warming the blood are essential steps in this procedure. Hot agar can damage red blood cells, and cold blood can cause the agar to gel before pouring [15,16].
2-Dilute 1 ml of bacterial isolate tube with 10 ml of deionized water into plane test tube and vibrate it with vortex for 30 second to spread the bacteria into all the diluted broth ; after that Rotate the swab against the side of the tube while applying pressure to remove excess liquid from the swab prior to inoculating the plate.
3-(A) Inoculate the plate with the test organism by streaking the swab in a back-and-forth motion very close together as you move across and down the plate. Rotate the plate 60° and repeat this action. Rotate the plate once more and repeat the streaking action. This ensures an even distribution of inoculum that will result in a confluent lawn of growth.
3-(B) Diagram illustrating the pattern the swab should follow as it is drawn across the plate.
4-Preparation of well in Blood Agar plates
First we divide the blood agar plate into four quadrant, at each quadrant about 6 mm in diameter well have been prepared by using sterile Pasteur pipette to cut through the blood agar media plate then remove the circular cut by sterile loop (Figure 2).
Figure 2. Blood agar plate
5- Preparation of ginger extract suspension by dissolving the ginger extract powder into sterile deionized water into test tube and mix it by vortex mixer until the ginger extract powder completely dissolved; the ginger extract suspension diluted to three percentages (25%,50% and 75%).
6-Fill the four wells at clock wise pattern respectively with:
• Control group (deionized water).
• 1st study group ( 25% aqueous ginger extract).
• 2nd study group ( 50% aqueous ginger extract).
• 3rd study group ( 75% aqueous ginger extract).
7-Placing the inoculated blood agar plates into anaerobic jar with anaerobic gaz pack for and incubated for 24 hours at 37 °C.
8-After that we checked the bacterial growth and the inhibition zone around the wells for bacterial isolate.
Results
The results in (Table 1) showed that the ginger extracts were effective against anaerobic flora of microorganisms used in this study, and the (75%) concentration had the best antimicrobial effect among the different dilutions of aqueous ginger extract of with the zone of inhibition zone length (mean value) (2.74) mm , and there is an increase of inhibition zone length with increase of ginger extract concentration as shown in Figures 3 and 4.
| Concentration of ginger extract | Inhibition zone length ( mean value) in mm |
|---|---|
| control | 0 |
| 25% | 0.747 |
| 50% | 1.574 |
| 75% | 2.74 |
Table 1: Antimicrobial activity of aqueous ginger extract using disc diffusion test.
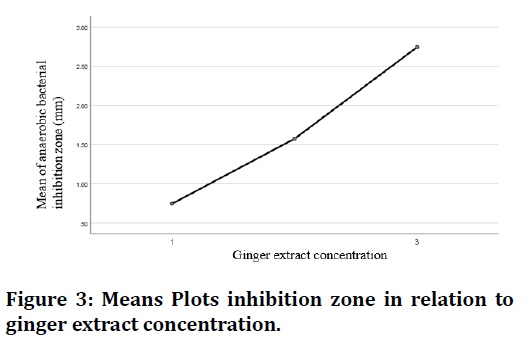
Figure 3. Means Plots inhibition zone in relation to ginger extract concentration.
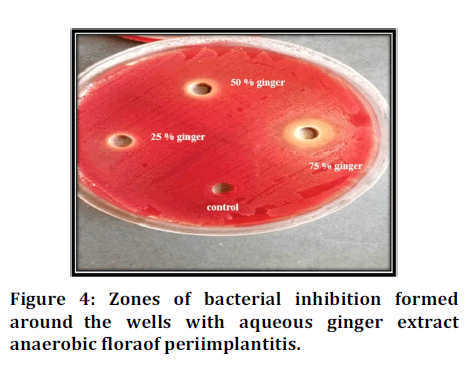
Figure 4. Zones of bacterial inhibition formed around the wells with aqueous ginger extract anaerobic flora of periimplantitis.
The statistical analysis shows significant differences between groups since (P< 0.05).
Discussion
Development of natural antimicrobials to control microbial diseases now a day become of concern for many population seeking for health care. The most commonly used natural antimicrobial agents are medicinal plants and spice has been used traditionally for thousands of years by many cultures for controlling common health complications. Antimicrobials drug discovery based on natural plant product are likely to be effective against multi drug resistant microbes [17].
This study was done to determine the antimicrobial effect of aqueous ginger extract against total anaerobic flora from patients with periimplantitis.
The inhibition zone of bacterial culture begins with concentration 25% and increased by raising the concentration of extract. This result agree with [18] who found that ginger extract has antibacterial effect against the cultures of E. Coli, Bacillus subtilis, Staphylococcus aureus and Streptococcus faecalis .
Effectiveness of ginger against different conditions attributed to its different constituents (volatile oils, shogaols, gingerols and diarylheptanoids) that show their therapeutic efficacy by modulating the genetic or metabolic activities of our body.
Conclusion
Our study shows that the aqueous ginger extract has a good antimicrobial effect against total anaerobic flora from periimplantitis, and the antimicrobial effect is promoted with the increase of ginger extract concentration and the best one is 75% concentration.
Acknowledgment
The authors would like to thank the university, Saveetha Institute of Medical and Technical Sciences, Saveetha Dental College and Hospitals for providing the required necessities for the present study.
Funding
The present project is supported/ funded/ sponsored by Saveetha Institute of Medical and Technical Sciences, Saveetha Dental College and Hospitals, Saveetha University.
Conflict of Interest
The authors declare that there were no conflicts of interest in the present study.
References
- Azu NC, Onyeagba RA. Antimicrobial properties of extracts of Allium cepa (Onions) and Zingiber officinale (Ginger) on Escherichia coli, Salmonella typhi, and Bacillus subtilis. Int J Trop Med 2007; 3:1-0.
- Atai Z, Atapour M, Mohseni M. Inhibitory effect of ginger extract on Candida albicans. Am J Applied Sci 2009; 6:1067-9.
- Chen IN, Chang CC, Ng CC, et al. Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Foods Human Nutri 2008; 63:15-20.
- Ficker CE, Smith ML, Susiarti S, et al. Inhibition of human pathogenic fungi by members of Zingiberaceae used by the Kenyah (Indonesian Borneo). J Ethnopharmacol 2003; 85:289-93.
- Chairgulprasert V, Prasertsongskun S, Wichaporn W. Chemical constituents of the essential oil and antibacterial activity of Zingiber wrayi var. halabala. Songklanakarin J Sci Technol 2005; 27:813.
- Ficker C, Smith ML, Akpagana K, et al. Bioassay‐guided isolation and identification of antifungal compounds from ginger. Phytotherapy research. Int J Pharmacol Toxicol Eva Natural Product Deri 2003; 17:897-902.
- Park M, Bae J, Lee DS. Antibacterial activity of [10]‐gingerol and [12]‐gingerol isolated from ginger rhizome against periodontal bacteria. Phytotherapy research. Int J Pharmacol Toxicol Eva Natural Product Deri 2008; 22:1446-9.
- Hiserodt RD, Franzblau SG, Rosen RT. Isolation of 6-, 8-, and 10-Gingerol from Ginger Rhizome by HPLC and Preliminary Evaluation of Inhibition of Mycobacterium a vium and Mycobacterium tuberculosis. J Agri Food Chem 1998; 46:2504-8.
- Nikolić M, Vasić S, Đurđević J, et al. Antibacterial and anti-biofilm activity of ginger (Zingiber officinale (Roscoe)) ethanolic extract. Kragujev J Sci 2014; 129-36.
- Ali BH, Blunden G, Tanira MO, et al. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food and Chem Toxicol 2008; 46:409-20.
- Tan BK, Vanitha J. Immunomodulatory and antimicrobial effects of some traditional Chinese medicinal herbs: A review. Curr Medi Chem 2004; 11:1423-30.
- Reddy YA, Chalamaiah M, Ramesh B, et al. Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J Food Sci Tech 2014; 51:908-14.
- Resnik R, Misch CE. Misch's avoiding complications in oral implantology. Elsevier 2018.
- Kannappan S, Jayaraman T, Rajasekar P, et al. Cinnamon bark extract improves glucose metabolism and lipid profile in the fructose-fed rat. Singapore Med J 2006; 47:858.
- Yin D, Guo Y, Li M, et al. Performance of VITEK 2, E-test, Kirby–Bauer disk diffusion, and modified Kirby–Bauer disk diffusion compared to reference broth microdilution for testing tigecycline susceptibility of carbapenem-resistant K. pneumoniae and A. baumannii in a multicenter study in China. Eur J Clin Microbiol Infect Dis 2021; 40:1149-54.
- Buxton R. Blood agar plates and hemolysis protocols. Am Soc Microbio 2005; 1-9.
- Karuppiah P, Rajaram S. Antibacterial effect of Allium sativum cloves and Zingiber officinale rhizomes against multiple-drug resistant clinical pathogens. Asian Pac J Trop Biomed 2012; 2:597-601.
- Riaz H, Begum A, Raza SA, et al. Antimicrobial property and phytochemical study of ginger found in local area of Punjab, Pakistan. Int Curr Pharmaceu J 2015; 4:405-9.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ahmed Ghaith Ahmed1 and Abdalbseet A Fatalla2*
1Department of Prosthodontics, College of dentistry, University of Baghdad, Baghdad, Iraq2Department of Prosthodontics, College of Dentistry, Al-Farahidi University, Baghdad, Iraq
Citation: Ahmed Ghaith Ahmed, Abdalbseet A Fatalla, Evaluation of the Anti-Bacterial Effect of Ginger Aqueous Extract on Bacterial Flora of Patient with Periimplantitis, J Res Med Dent Sci, 2022, 10(1): 570-574
Received: 23-Dec-2021, Manuscript No. JRMDS-21-45555; , Pre QC No. JRMDS-21-45555 (PQ); Editor assigned: 27-Dec-2021, Pre QC No. JRMDS-21-45555 (PQ); Reviewed: 10-Jan-2022, QC No. JRMDS-21-45555; Revised: 13-Jan-2022, Manuscript No. JRMDS-21-45555 (R); Published: 20-Jan-2022
