Research - (2019) Volume 7, Issue 4
Evaluation of the Antibacterial Efficacy of Silver Nanoparticles as an Irrigant against Enterococcus faecalis In vitro Study
Batool M AL-Fhham and Aseel Haidar MJ AL-Haidar*
*Correspondence: Aseel Haidar MJ AL-Haidar, Department of Pedodontics and Preventive Dentistry, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Background: Successful root canal therapy depends on thorough chemo mechanical debridement of pulpal tissue, dentin debris and infective microorganisms.
Objective: This study aimed to investigate the antibacterial effect of silver nanoparticles, sodium hypochlorite and chlorhexidine in reducing the bacterial infection of the root canals.
Materials and Methods: The root canals of 55 single-rooted teeth were cleaned, shaped, and sterilized. All the teeth samples were inoculated with Enterococcus faecalis and incubated at 37°C for 2 weeks. Then, the teeth were divided into four groups. Group I (n=15): 100 ppm silver nanoparticles, Group II (n=15): 2.5 sodium hypochlorite, Group III (n=15): 2% chlorhexidine, IV (n=10): Normal saline as a control group. Specimens were incubated for 2 weeks. Paper points were used to obtain pre- and post-irrigation samples so that the colony-forming units were counted. Data were analyzed using SPSS and tested by Shapiro-Wilk test, One-Way ANOVA and Games-Howell test where the level of significance was set at 0.05.
Results: All the tested irrigants showed superior effectiveness compared to the normal saline (p<0.05). Overall, 2.5% sodium hypochlorite presented the most effective action against E. faecalis biofilm, followed by 100 ppm silver nanoparticles, then the 2% chlorhexidine by mean percentage of antibacterial effectiveness of 99.87%, 99.51% and 98.66% respectively.
Conclusions: Silver nanoparticles were effective against E. faecalis biofilm similarly to sodium hypochlorite when it was used as an irrigation solution.
Keywords
Antibacterial, Chlorhexidine, Enterococcus faecalis, Silver nanoparticles, Sodium hypochlorite
Introduction
Successful endodontic treatment depends upon various factors such as chemomechanical debridement, optimum irrigation, appropriate and specific intracanal medicaments and three-dimensional obturation in order to get a complete seal of the root canal system. The complicated anatomy of the root canal system permits the survival of microorganism after the completion of standard cleansing [1].
Enterococcus faecalis is a normal inhabitant of the oral flora, which is facultative anaerobic microorganism, Grampositive and related, most often, with the failure of the root canal treatment also as in several kinds of periradicular lesions including primary and secondary endodontic infections [2,3]. It is capable for extant within the harsh nutritional conditions and may keep viable as a one microorganism [4]. It has the ability of dentinal tubule penetration and biofilm formation [5].
Various irrigants at variable concentrations such as sodium hypochlorite (NaOCl) and chlorhexidine (CHX) are used as an adjunct to conventional mechanical root canal cleaning methods [6]. However, root canal irrigants are only effective when they come in contact with the surface, and because of the anatomical barriers they cannot penetrate deep into the surfaces [7]. NaOCl able to penetrate the dentinal tubules into 130 mm, whereas bacteria able to penetrate into 1000 mm [8]. In addition, irrigation with sodium hypochlorite leads to decreased bond strength between dentin and resin [9]. As well as CHX has a lower ability to dissolve bacterial biofilms compared with sodium hypochlorite [10,11].
Due to this, the efforts of researchers in the endodontic field focused in developing a new nanomaterial-based irrigant [12].
Nanotechnology is considered to be a breakthrough in the field of medicine. It is helpful in manufacturing enhance biomaterials with distinctive physical, chemical and biological properties [13,14]. This is chiefly approached by enhancing surface-to-volume ratio. To regulate numerous biological processes, nanomaterials can be used with predefined geometries, surface characteristics and mechanical strength [15].
Silver nanoparticles (AgNPs) are effective against several microorganisms including E. faecalis, their use in disinfection attributed to their optimum antibacterial properties. They possesses high surface area and positive charge density and polycationic/polyanionic properties that enhance the antibacterial effect [5,16]. It is suggested to use AgNP solution as an alternative to endodontic irrigating solution not just for its robust bactericidal activity but additionally for its biocompatibility, particularly in low concentrations [17].
The aim of this study was to determine the efficacy of Silver nanoparticles in reducing the bacterial infection in the root canal when used as an irrigant compared to sodium hypochlorite and chlorhexidine.
Materials and Methods
Teeth selection and preparation
Fifty-five single rooted permanent teeth, extracted for orthodontic reasons, were collected from children younger than fifteen years old. Teeth with root caries and canal calcifications were excluded from the study. Soft tissues, calculus and bone on the surfaces of the root were slightly removed by periodontal curette. Then placed in 5.25% NaOCl for 1 hour to disinfect the root surface after that the teeth stored in a normal saline [18]. Roots were cut at 14-mm distance from the apex employing diamond disc.
The working length was 13 mm (1 mm from the apical foramen). Preparation of the canals was done by the using of ProTaper rotary files (CICADA) up to F4. Then canals were irrigated with 2.5% NaOCl between the use of the rotary files, using a 5 ml syringe and endodontic needle. Once completion of the preparation, each canal were irrigated with 1 ml 17% EDTA, 5 ml normal saline and 1 ml NaOCl, respectively, each one for 3 minutes employing endodontic needle to get rid of the smear layer. Finally, all the canals were irrigated with 5 ml saline solution. The root surfaces were coated with 2 layers of a nail varnish after sealing the apical foramen with chemical-cure glass ionomer. Then the teeth were put in screw cup glasses and autoclaved for 30 minutes at 12°C with 15 IB [19].
Bacterial isolation
E. faecalis were isolated from the infected root canals. Several samples were taken from chronic infected roots that were suspected to be inhabitant with E. faecalis. Bile esculin test (selective test for E. faecalis) was used for the first identification of the bacteria where black deposit appears after 24 hours of the samples culturing [20]. Then E. faecalis were further detecting by Vitek 2 system for more accuracy [21].
Biosynthesis of silver nanoparticles
AgNps was synthesized from (probiotic) of the bacillus mixture by adding Silver nitrate (AgNO3) to cell free supernatant that obtained from the centrifuge the incubated broth of bacillus, after that it incubated in shaker incubator and centrifuged, the supernatant was discarded and the deposit at the bottom of the tube which represent the collection of the nanoparticles. All the steps were done in dark condition to avoid oxidation of AgNO3 [22-24]. Characterization of the Ag Nanoparticles was analyzed using the ultraviolet (UV)- visible spectroscopy (Figure 1), Scanning electron microscope (SEM) (Figure 2), Atomic force microscope (AFM) and X-ray diffraction (XRD). It has spherical shape and Avg. Diameter: 33.53 nm.
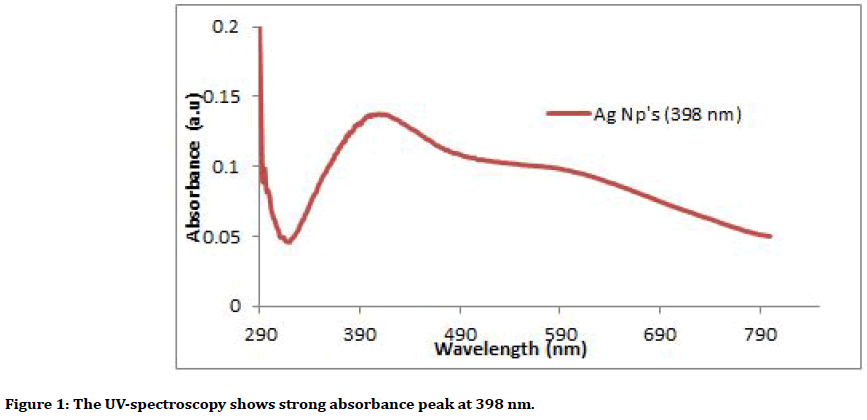
Figure 1. The UV-spectroscopy shows strong absorbance peak at 398 nm.
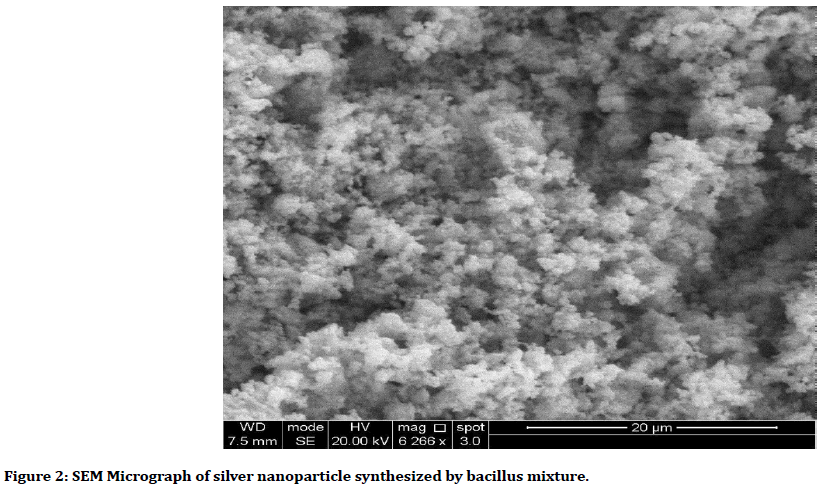
Figure 2. SEM Micrograph of silver nanoparticle synthesized by bacillus mixture.
A 24 hour pure culture of E. faecalis was suspended in 5 ml of brain/heart infusion broth (BHI), then it incubated for 4 hour at 37ºC [25]. The sterilized tooth specimens were inoculated with 2 ml of 0.5 Mc Farland solution of the bacterial suspension that was inject into each canal using sterile needle then incubated at 37°C [12]. Two weeks period was chosen for the inoculation of bacteria. All the microbiological procedures were done under the sterile environment in a laminar flow hood [26].
Antimicrobial activity of the irrigants
The teeth were removed from the screw cup glass aseptically, after incubation period, and gently rinsed with sterile saline to remove the culture medium. Then, a pre irrigation sample (S1) was obtained by sterilized #25-paper point applied into the root canal for 1 minute [27]. The samples were randomly divided into 4 groups for the experimental procedure of the irrigation materials.
Irrigation procedure
Group I (n=15): 5 ml of 100 ppm AgNPs was inserted into each root canal followed by irrigation with normal saline.
Group II (n=15): 5 ml of 2.5% NaOCl was used followed by irrigation with normal saline.
Group III (n=15): 5 ml of 2% CHX was irrigated into each root canal followed by irrigation with normal saline.
Group IV (n=10): root canals were irrigated with 5 ml of normal saline as a control group.
The irrigation time was 5 minutes. for each sample. Each solution was delivered into the canal lumen with sterile 5 ml plastic syringes and endodontic needles. The same operator performed all the irrigation procedures at room temperature under aseptic conditions [12].
Then bacteriological samples were obtained using sterile paper points (S2). Both the pre and the post sample were kept in Eppendorf tubes which containing 1 ml of normal saline and vortexed for 30 seconds each [27]. After that serial dilution were done. Then, each dilution were pipetted and cultured on blood agar plates and incubated for 24 hour at 37°C. The colony forming units (CFU) counted and calculated to give CFU/ml (Figure 3) [28].
Figure 3. The colony forming units that were grown on blood agar.
Then bacteriological samples were obtained using sterile paper points (S2). Both the pre and the post sample were kept in Eppendorf tubes which containing 1 ml of normal saline and vortexed for 30 seconds each [27]. After that serial dilution were done. Then, each dilution were pipetted and cultured on blood agar plates and incubated for 24 hour at 37°C. The colony forming units (CFU) counted and calculated to give CFU/ml (Figure 3) [28].
Results
Table 1 showed that there was a reduction in the means of the post samples of the bacterial colonies for all the four methods, with the largest effect size was to the NAOCL group, followed by AgNps group then CHX group while the least effect size was to the normal saline group. Meanwhile, the effectiveness of each sample was calculated according to equation reported by Dunavant et al. [29]. Sodium hypochlorite showed the largest mean percentile of the antibacterial effectiveness followed by of silver nanoparticles then chlorhexidine while the lowest percentage was found for the normal saline (Table 2). A significant difference was found between the antibacterial efficacy of the four groups (p=0.000), as shown in Table 3.
| Paired Samples Statistics | Paired t- test | p value | Effect Size | ||||
|---|---|---|---|---|---|---|---|
| Group | Before | After | |||||
| Mean | SE | Mean | SE | ||||
| Saline | 3996875 | 1303913.757 | 1673375 | 551261.371 | 2.959 | 0.01 | 0.764 |
| Silver | 7439916.667 | 2146129.158 | 8950 | 4317.062 | 3.46 | 0.004 | 0.97 |
| NAOCL | 4066166.667 | 1050299.408 | 750 | 433.013 | 3.87 | 0.002 | 0.999 |
| CHX | 5387500 | 1808611.235 | 34033.3 | 15709.337 | 3.067 | 0.013 | 0.893 |
Table 1: The paired samples statistics of effective size for pre and post Samples for all groups.
| Groups | N | Mean | ± SD | ± SE | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Saline | 10 | 58.1445 | 3.3724 | 1.0664 | 53.8994 | 62.9723 |
| Silver | 15 | 99.514 | 1.0904 | 0.2815 | 95.9857 | 100 |
| NAOCL | 15 | 99.8702 | 0.2641 | 0.0682 | 99.0909 | 100 |
| CHX | 15 | 98.669 | 1.9202 | 0.4958 | 95.0456 | 100 |
Table 2: The antibacterial effectiveness of the four irrigant methods used in the study.
| ANOVA | |||||
|---|---|---|---|---|---|
| Groups | Sum of Squares | df | Mean Square | F | Sig. |
| Between Groups | 13901.906 | 3 | 4633.969 | 1377.241 | 0 |
| Within Groups | 171.598 | 51 | 3.365 | - | - |
| Total | 14073.504 | 54 | - | - | - |
| Levene Statistic=24.637, df=3, p=0.000 | |||||
Table 3: Statistical test of Effect (One-Way ANOVA analysis) among irrigants.
However, the multiple comparisons between the groups by Games-Howell showed that there was a significant difference between the normal saline and the other three groups, p=0.000. While the comparison between Silver nanoparticles, NAOCL and CHX showed that there was no significant difference between them (Table 4).
| Multiple Comparisons by Games-Howell | |||||
|---|---|---|---|---|---|
| (I) Group | (J) Group | Mean Difference (I-J) | Sig. | 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||||
| Saline | Silver | -41.3694 | 0 | -44.728 | -38.0109 |
| NAOCL | -41.7256 | 0 | -45.0561 | -38.3951 | |
| CHX | -40.5144 | 0 | -43.9691 | -37.0598 | |
| Silver | NAOCL | -0.3562 | 0.6182 | -1.1871 | 0.4747 |
| CHX | 0.855 | 0.4545 | -0.7272 | 2.4372 | |
| NAOCL | CHX | 1.2112 | 0.117 | -0.2368 | 2.6591 |
Table 4: Comparison between the antibacterial effects of the irrigants methods.
Discussion
Mechanical cleansing and irrigation with antimicrobial potential solutions is the essential steps for root canal disinfection [30]. Enterococcus faecalis is the predominant microorganism related to persistent periradicular lesions, which is occasionally detected as the sole microorganism in root canals of the teeth [31]. This microorganism has special virulence factors including cytolysin, lytic enzymes, aggregation substance, lipoteichoic acid, and pheromones [32]. E. faecalis is ready to invade dentinal tubules and stay viable inside the tubules for extend period of time resist the intracanal disinfectants [33]. Sodium hypochlorite is the most counseled root canal irrigant, attributed to its antimicrobial effectiveness and tissue dissolution capability [34]. Even so, the direct employing of sodium hypochlorite is potentially harmful as related to cellular destruction of the tissues [19,35]. To enhance the properties of the antibacterial agents utilized in endodontic treatment, innovative antimicrobial delivery systems had been developed, like nanoparticles [36]. Nanoparticles are outlined as particles with tiny sizes, massive surface/area mass ratio with external dimensions of 1 – 100 nm, and enhanced chemical reactivity [37,38]. The properties of nanoparticles as larger surface area and the charge density enable them to interact to a large degree with the negatively charged surface of the bacterial cells, leading to an increased antimicrobial activity [39]. Silver nanoparticles have the ability to attach and penetrate the cell walls of each Gram-positive and Gram-negative bacteria, releasing silver ions that lead to disturbance in the cell function. Therefore, they are used for inhibition of the biofilm formation and treatment and prevention of drugresistant microorganisms [37]. Nanoparticles have additionally studied in the field of endodontic in an effort to improve root canal disinfection [40] by minimizing E. faecalis adherence to dentine and biofilm elimination [39,41]. The result of the present study, showed a highly antibacterial effectiveness of the three types of the irrigation solutions (sodium hypochlorite, silver nanoparticles and chlorhexidine) which were 99.87, 99.51, 98.66 respectively. However, there was a significance difference between them. Meanwhile, normal saline showed the least antimicrobial effect (58.14). The activity of silver nanoparticles in the elimination of E. faecalis that had been seen in this study was near that found with the use of NaOCl, and this result was in agreement with the findings of Luna et al. [12] who evaluated the effect of using AgNPs as a final irrigant on E. faecalis. They found a positive bactericidal effect from the AgNPs solution as an endodontic irrigation with the same effect occurred by using sodium hypochlorite at 2.25%, so that these nanoparticles were the good choice to get rid E. faecalis in the root canal treatment. In addition, the finding of the present study was in accordance with that found by other study done by Moghadas et al. [42]. They found that this nanosilverbased root canal irrigant was an effective agent for eradication of E. faecalis and S. aureus and there was no significant difference between NaOCl and the nanosilverbased irrigant, so as this in vitro study. Meanwhile, Afkhami et al. [19] also showed that AgNPs at 100 ppm concentration had an efficacy similar to the use of 2.5% NaOCl in the reduction of bacterial count. However, de Almeida et al. [43] demonstrated that AgNPs solutions had similar antimicrobial activity compared to the conventional endodontic irrigants (sodium hypochlorite and chlorhexidine) p>0.05. On the other hand, the results of the current study was in disagreement with other studies [44,45]. Wu et al. [44] highlighted that irrigation with 0.1% AgNP solution did not disrupt E. faecalis biofilm structure or produce significant killing of the resident biofilm bacteria. While Rodrigues et al. [45] demonstrated that the 94 ppm silver nanoparticle solution tested was not effective in disrupting E. faecalis biofilm when compared with NaOCl and the AgNP solution was significantly less effective (p<0.05) than chlorhexidine in killing bacteria in biofilms when irrigated for 5 min. The explanation for this disparity in the results may be related to the differences in the methodology used and variance in the microbial strains that had been tested.
Conclusion
Silver nanoparticles were effective as 2.5% NaOCl and 2% chlorhexidine against E. faecalis, so it can be used as an alternative to the NaOCl. Further in vitro and in vivo studies are essential to validate the use of Silver nanoparticles as an irrigant against E. faecalis.
References
- Paredes-Vieyra J, Enriquez FJ. Success rate of single- versus two-visit root canal treatment of teeth with apical periodontitis: A randomized controlled trial. J Endod 2012; 38:1164-1169.
- Haapasalo M, Endal U, Zandi H, et al. Eradication of endodontic infection by instrumentation and irrigation solutions. Endodontic Topics 2005; 10:77–102.
- Athanassiadis B, Abbott PV, Walsh LJ. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent J 2007; 52:64–82.
- Zhang C, Du J, Peng Z. Correlation between Enterococcus faecalis and persistent intraradicular infection compared with primary intraradicular infection: a systematic review. J Endod 2015; 41:1207–1213.
- Du T, Wang Z, Shen Y, et al. Effect of long-term exposure to endodontic disinfecting solutions on young and old Enterococcus faecalis biofilms in dentin canals. J Endod 2014; 40:509–14.
- Rocas IN, Siqueira JF. Comparison of the in vivo antimicrobial effectiveness of sodium hypochlorite and chlorhexidine used as root canal irrigants: A molecular microbiology study. J Endod 2011; 37:143–50.
- Mehrvarzfar P, Saghiri MA, Asatourian A, et al. Additive effect of a diode laser on the antibacterial activity of 2.5% NaOCl, 2% CHX and MTAD against Enterococcus faecalis contaminating root canals: an in vitro study. J Oral Sci 2011; 53:355–60.
- Rios A, He J, Glickman GN, et al. Evaluation of photodynamic therapy using a lightemitting diode lamp against Enterococcus faecalis in extracted human teeth. J Endod 2011; 37:856–859.
- Morgental RD, Singh A, Sappal H, et al. Dentin inhibits the antibacterial effect of new and conventional endodontic irrigants. J Endod 2013; 39: 406–410.
- Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J 2009; 42:288–302.
- Del Carpio-Perochena AE, Bramante CM, Duarte MA, et al. Biofilm dissolution and cleaning ability of different irrigant solutions on intraorally infected dentin. J Endod 2011; 37:1134–8.
- González-Luna PI, Martinez-Castanon GA, Zavala-Alonso NV, et al. Bactericide effect of silver nanoparticles as a final irrigation agent in endodontics on enterococcus faecalis: An ex vivo study. J Nanomater 2016; 1:1-7.
- Thomas J, Peppas N, Sato M et al. Nanotechnology and biomaterials. Boca Raton: CRC Taylor and Francis.
- Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release 2014; 173:75-88.
- Xavier JR, Thakur T, Desai P, et al. Bioactive nanoengineered hydrogels for bone tissue engineering: A growth-factorfree approach. ACS Nano 2015; 9:3109-3118
- Javidi M, Afkhami F, Zarei M, et al. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust Endod J 2014; 40:61–5.
- Gomes-Filho JE, Silva FO, Watanabe S, et al. Tissue reaction to silver nanoparticles dispersion as an alternative irrigating solution. J Endod 2010; 36:1698–702.
- Mozayeni MA, Haeri A, Dianat O et al. Antimicrobial effects of four intracanal medicaments on enterococcus faecalis: An in Vitro Study. Iranian Endod J 2014; 9:195-198.
- Afkhami F, Akbari S, Chiniforush N. Entrococcus faecaliselimination in root canals using silver nanoparticles,photodynamic therapy, diode laser, or laser-activatednanoparticles: an in vitro study. J Endod 2017; 43:279-282.
- Mahmoudpour A, Rahimi S, Sina M, et al. Isolation and identification of Enterococcus faecalis from necrotic root canals using multiplex PCR. J Oral Sci 2007; 49:221-227.
- Garcia-Garrote F, Cercenado E, Bouza E. Evaluation of a new system, VITEK 2, for identification and antimicrobial susceptibility testing of enterococci. J Clin Microbiol 2000; 38:2108–2111.
- Chaudhari PR, Masurkar SA, Shidore VB et al. Antimicrobial activity of extracellularly synthesized silver nanoparticles using Lactobacillus species obtained from vizylac capsule. J Applied Pharma Sci 2012; 02:25-29.
- Sarvamangala D, Kondala K, Shivakumar N, et al. Synthesis characterization and antimicrobial studies of AgNP’s using probiotics. Int Res J Pharm 2013; 4:240-243.
- Maria BS, Devadiga A, Kodialbail VS et al. Synthesis of silver extract nanoparticles using medicinal Zizyphus xylopyrus bark extract. Appl Nanosci 2015; 5:755–762.
- Chandra A,Yadav RK, Shakya VK, et al. Antimicrobial efficacy of silver nanoparticles with and without different antimicrobial agents against enterococcus faecalis: Ex Vivo Study. J Dent Oral Biol 2017; 2:1047.
- Sohrabi K, Sooratgar A, Zolfagharnasab K, et al. Antibacterial activity of diode laser and sodium hypochlorite in enterococcus faecalis-contaminated root canals. Iranian Endod Journal 2016; 11:8.
- Alabdulmohsen ZA, Saad AYS. Antibacterial effect of silver nanoparticles against Enterococcus faecalis. Saudi Endod J 2017; 7:29-35.
- Radwan IN, Randa B, Hend AN, et al. Evaluation of antimicrobial efficacy of four medicinal plants extracts used as root canal irrigant on Enterococcus faecalis: An in-vitro study. Int Dent Med J Adv Res 2015; 1;1-8.
- Dunavant TR, Regan JD, Glickman GN, et al. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J Endod 2006; 32:527-531.
- Nair PN, Henry S, Cano V, et al. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; 99:231–252.
- Stuart CH, Schwartz SA, Beeson TJ, et al. Enterococcus faecalis: its role in rootcanal treatment failure and current concepts in retreatment. J Endod 2006; 32:93–98
- Rocas IN, Siqueira JF, Santos KRN. Association of Enterococcus faecalis with different forms of periradicular diseases. J Endod 2004; 30:315–320.
- Love RM. Enterococcus faecalis: a mechanism for its role in endodontic failure. Int Endod J 2001; 34:399–405.
- Haapasalo M, Shen Y, Qian W, et al. Irrigation in endodontics. Dent Clin North Am 2010; 54:291–312.
- Bramante CM, Duque JA, Cavenago BC, et al. Use of a 660-nm laser to aid in the healing of necrotic alveolar mucosa caused by extruded sodium hypochlorite: a case report. J Endod 2015; 41:1899–902.
- Samiei M, Farjami A, Dizaj SM, et al. Nanoparticles for antimicrobial purposes in Endodontics: a systematic review of in vitro studies. Mater Sci Eng C Mater Biol Appl 2016; 58:1269–78.
- Rai MK, Deshmukh SD, Ingle AP, et al. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J Applied Microbiol 2012; 112:841–852.
- Shrestha A, Kishen A. Antibacterial nanoparticles in endodontics: A review. J Endod 2016; 42:1417–1426.
- Kishen A, Shi Z, Shrestha A, et al. An investigation on the antibacterial and antibiofilm efficacy of cationic nanoparticulates for root canal disinfection. J Endod 2008; 34:1515–1520.
- Shrestha A, Fong SW, Khoo BC, et al. Delivery of antibacterial nanoparticles into dentinal tubules using high-intensity focused ultrasound. J Endod 2009; 35:1028–33.
- Shrestha A, Shi Z, Neoh KG, et al. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J Endod 2010; 36:1030-1050.
- Moghadas L, Narimani T, Shahmoradi M. Antimicrobial activity of a new nanobased endodontic irrigation solution: In vitro study. Dent Hypotheses 2012; 3:142-146.
- Almeida J, Cechella BC, Bernardi AV, et al. Effectiveness of nanoparticles solutions and conventional endodontic irrigants against Enterococcus faecalis biofilm. Indian J Dent Res 2018; 29:347-351.
- Wu D, Fan W, Kishen A, et al. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J Endod 2014; 40:285–290.
- Rodrigues CT, de Andrade FB, de Vasconcelos LRSM, et al. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int Endod J 2018; 51:901-911.
Author Info
Batool M AL-Fhham and Aseel Haidar MJ AL-Haidar*
Department of Pedodontics and Preventive Dentistry, College of Dentistry, University of Baghdad, IraqCitation: Batool M ALfhham, Aseel Haidar MJ AL-Haidar, Evaluation of the Antibacterial Efficacy of Silver Nanoparticles as an Irrigant against Enterococcus faecalis In vitro Study, J Res Med Dent Sci, 2019, 7(4): 21-27.
Received: 27-Jul-2019 Accepted: 20-Aug-2019
