Research - (2022) Volume 10, Issue 3
Fluoride Release and Solubility of New Bioactive Restoratives used in Pediatric Dentistry Part 1 (Fluoride Release)
Shukran Abdalhasan Mohamad Samir* and Baydaa Hussein
*Correspondence: Shukran Abdalhasan Mohamad Samir, Department of Pedodontics and Preventive Dentistry, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
The goal of this in vitro study was to compare and measure fluoride ion release from a variety of innovative bioactive materials, including very viscous glass ionomer cement (EQUIA Forte HT), resin modified glass ionomer material (activa KIDS bioactive), and composite resin (predicta pulk bioactive). The test materials were created as molds with specific dimensions, and each disc was placed in a vial containing 15 ml of deionized Distal Water. The amount of fluoride emitted across two time periods was measured (7day, 28days). Ion selective electrodes (ISE) were used to determine fluoride release data. The GIC (highly viscous glass ionomer) had a larger release than the other two materials, followed by (active kids bioactive) and then the composite resin (predicta bulk bioactive), with significant differences between them at P<0.05. In comparison to the predicta bulk bioactive, the active kids exhibited higher fluoride ions release concentrations. The CGI, on the other hand, showed larger fluoride ion release concentrations than the other two materials.
Keywords
Pediatric dentistry, Fluoride release, Enamel, Bioactive
Introduction
In dental clinics, recurrent caries is the most common reason of restorative failure. Industrialized dental materials are still trying to figure out how to make their compositions less likely to cause recurrent caries. Over the last two decades, fluoride-containing restorative materials have gotten a lot of attention. Fluoride reduces caries activity by acting as a biocide and decreasing the solubility of enamel and dentin by forming fluoroapatite when it is absorbed into tooth tissue. Furthermore, it has been discovered that Following demineralization, fluoride assists in the remineralization of injured tooth tissue [1,2].
Fluoride loss was seen in varying degrees in all fluoridated dental materials over time [3]. In most situations, fluoride is present in these materials in the form of NaF, CaF2, SnF2, KPF6, YbF3, or fluoro-alumino-silicate glass.. Because of the differences in solubility, the amount of fluoride released by each chemical varies. Many factors influence the pace and pattern of fluoride ion release from restorative materials, including the structure of the components, the temperature, the mixing process, the powder liquid ratio, the pH of the surrounding environment, and the exposed part to the oral environment [4]. These materials are commonly employed as fluoride reservoirs because they may be recharged with fluoride from a current source [5].
The glass ionomer was a well-known restorative filling material.
The release of fluoride ions is one of the benefits of the glass ionomer. The poly-acid assaults the glass, causing cations and fluoride ions to be released [4,5]. Fluoride ions are released in proportion to the concentration of fluoride ions that can permeate through the repair surface from the matrix and/or residual silicate particles. Fluoride ion release is often rapid during the first few days, but as the fluoride concentration of the matrix diminishes, so does the rate of release.
GIC is a bioactive substance that uses ionic bonding methods to chemically attach to hard tooth tissues. Furthermore, the fluorine in These substances promote remineralization and the elimination of carious lesions [2,5]. Glass ionomer cements are widely employed in dental medicine because to a variety of properties, including low Toxicity and high biocompatibility, great adhesion to enamel and dentin, pulp protection, reduced volumetric shrinkage, and fluoride ion release are all advantages of this material. (i.e., caries prevention) [6,7].
In 2007, GC Corporation (Tokyo, Japan) presented a novel restorative concept that included two components: Fuji IX GP Extra (GC, Tokyo, Japan) glass ionomer with a high viscosity and an Equia Fil nano-filled covering. With a protective covering packed with nanoparticles, better mechanical characteristics can be attained. It is partially absorbed into the material, which improves the material's visual features as well as its mechanical properties (using a micro laminated technique). The Equia Forte Fil glass hybrid materials (GC, Tokyo, Japan) were built on the Equia Fil platform in 2015, and the Equia Forte HT Fil material (GC, Tokyo, Japan) was made in 2019. Glass hybrid materials based on GIC technology have been developed by including glass particles of varying sizes into the standard filler, such as highly reactive tiny particles.
ACTIVA Kids Bioactive restoratives are ionic composite resins that combine GIC's biocompatibility, chemical bond, and fluoride release ability with RBC's mechanical qualities, appearance, and durability. Furthermore, those materials are said to have bioactive qualities, since The claim that ACTIVA Bioactive products have a bioactive matrix and bioactive fillers, distinguishing them from the other toothcolored restorative materials described, has been authorized by the US Food and Drug Administration [8-10].
Predicta™ Bioactive Bulk materials are bulk-fill resin composites that are dual-cured. Predicta Bioactive Bulk is bioactive, releasing fluoride, calcium, and phosphate ions at the material-tooth contact to promote mineral apatite production and remineralization. Assume the manufacturer's instructions. Stronger connections between the restoration and the tooth, penetration and filling of micro-gaps, decrease in sensitivity, protection against secondary caries, and sealing of margins against micro leakage and failure are all documented benefits of bioactivity in the field (Parkell, USA).
Due to their ion release and tooth remineralization, new bioactive restorative materials may help reduce the incidence of secondary caries and increase the success rate of restorative treatments, given the passivity of dental composites and the fact that they play no role in protecting restored teeth against secondary caries. The purpose of this study was to compare the release of fluoride ions from two bioactive materials to a novel bioactive composite resin.
The goal of this research was to determine how much fluoride was released from three restorative materials: GIC (equia forte/Gc), Activa kids composite material, and predicta bioactive bulk composite material. Also, to compare the fluoride ion release characteristics of the three restoratives listed above.
Material and Methods
Details of the restorative materials used in this study are mentioned in Table 1.
| Restorative | Type of Material | Composition according to manufacturer | Working & setting time according to manufacturer |
|---|---|---|---|
| EQUIA Forte® HT ,Bulk Fill Glass Hybrid Restorative | High viscosity glass-ionomer hybrid glass system | Powder: Strontium-fluoroaluminosilicate glass, polyacrylic acid, iron oxide Liquid: polybasic carboxylic acid, water | working time: (23 °C) 1’30”second |
| Net setting time: (37°C) 2’30” second | |||
| ACTIVA Kids BioACTIVE RESTORATIVE | Bioactive ionic resin with reactive glass filler . bioglass-reinforced glass ionomer restorative cement) | Working time at room temperature:90 seconds. | |
| Mix of methacrylates and diurethane with modified polyacrylic acid (44.6%); reactive glass filler (21.8 wt. %); inorganic filler (56 wt. %), patented rubberized resin (Embrace), water. (wt = weight percent) | Self-cure anaerobic setting time at 37°C: < 3 minute | ||
| Predicta™ Bulk BIOACTIVE dual-cured Restorative | Bulk fill resin composite | Limited information. | Work Time of Self-cure (25°C): 1-2 min. |
| Set Time of Self-cure (37°C): 5 min |
Table 1: Details of the restorative materials used in this study.
Sample preparation
A total of 60 discs are created from these three materials using custom-built moulds with a diameter of 3 mm and a thickness of 8 mm, as shown in Figure 1. To release any air bubbles and remove extra material, a clear acetate sheet with glass slabs was put below and above the material-filled moulds, as shown in Figure 2. The disk was then cured according to each material's manufacturer's specifications, as shown in Figure 3. The discs were completed with a hand instrument.
Figure 1: SP 1.
Figure 2: SP 2.
Figure 3: SP 2.
Fluoride releasing measurement
Each disk will then be immersed in a 15 ml securely sealed centrifuge tube containing 10 ml de ionized distilled water to remove any ions from the water, and the tubes will be kept in an incubator at 37±1˚C.
A fluoride ion selective electrode (ISE) MM 41 MultiMeter designed and manufactured by CRISON INSTRUMENTS, Made in EU, was connected to a digital ion analyzer to calculate fluoride releasing readings. The electrode was calibrated using a standard solution of NaF at successive dilutions of 10-1,10-2,10-3,10-4, and 10-5 ppm F-, as shown in figure(4). Each solution's concentration is shown in millivolts on the instrument display (mv). The measurement was then converted to molarity using the calibration curve, and then multiplied by 1000 times the atomic weight of fluoride to convert to ppm. (10) At 7 days and 28 days, the quantity of fluoride emitted was measured.
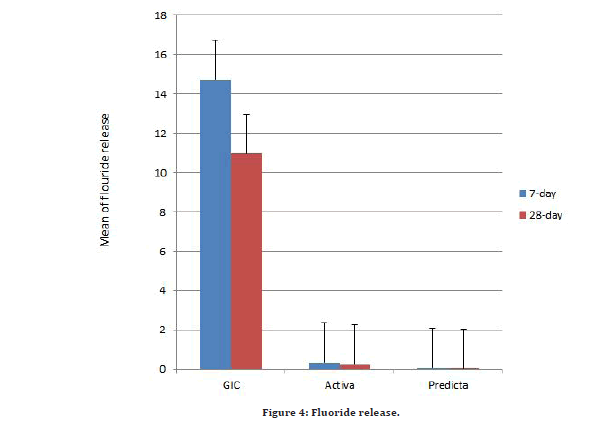
Figure 4:Fluoride release.
The Statistical Package for Social Science (SPSS version 21) was used to examine the data statistically (Chicago, Illinois, USA). The difference between k independent groups was tested using One Way Analysis Of Variance (ANOVA) and Dunnett T3 tests (Unequal variance). All tests had a p 0.05 significance level.
Results
Results in Table 2 show that in each group the fluoride release is higher in 7-day than that in 28-day with significant finding in GIC while not significant difference in other two groups.
| Groups | Storage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 7-day | 28-day | |||||||||
| Min. | Max. | Mean | ±SD | Min. | Max. | Mean | ±SD | T -test | P value | |
| GIC | 12.47 | 20.01 | 14.732 | 2.17 | 7.3 | 17.06 | 10.97 | 3.363 | 2.972 | 0.000* |
| Activa | 0.18 | 0.84 | 0.34 | 0.189 | 0.02 | 0.78 | 0.239 | 0.216 | 1.111 | 0.281 ^ |
| Predicta | 0.028 | 0.095 | 0.069 | 0.021 | 0.019 | 0.085 | 0.047 | 0.027 | 2.074 | 0.053^ |
Table 2: Descriptive and statistical test of fluoride release among storage period by groups.
Our findings show that equia has the most fluoride release in each storage period, followed by Activa, and Predicta have the least. There is a significant difference in each storage period, with the most difference found in the first week compared to the fourth week. Following that, after multiple comparisons, there is a significant difference in the first week when comparing each group to each other, while after four weeks, all results are significant except when comparing Activa with Predicta the result is not significant (Tables 3 and Table 4) (Figure 4).
| Storage | Groups | Min. | Max. | Mean | ±SD | F | P value |
|---|---|---|---|---|---|---|---|
| 7-day | GIC | 12.47 | 20.01 | 14.732 | 2.17 | 444.892 | 0.000* |
| Activa | 0.18 | 0.84 | 0.34 | 0.189 | |||
| Predicta | 0.028 | 0.095 | 0.069 | 0.021 | |||
| 28-day | GIC | 7.3 | 17.06 | 10.97 | 3.363 | 103.235 | 0.000* |
| Activa | 0.02 | 0.78 | 0.239 | 0.216 | |||
| Predicta | 0.019 | 0.085 | 0.047 | 0.027 | |||
| Df=2,*=significant at p<0.05 | |||||||
Table 3: Descriptive and statistical test of fluoride release among groups by storage periods.
| Multiple Comparisons of fluoride release among groups by storage periods using Dunnett T3 | ||||
| Storage | (I) Groups | (J) Groups | Mean Difference (I-J) | P value |
| 7-day | GIC | Active | 14.392 | 0.0000* |
| Predicta | 14.6627 | 0.0000* | ||
| Active | Predicta | 0.2707 | 0.0040* | |
| 28-day | GIC | Active | 10.731 | 0.0000* |
| Predicta | 10.9231 | 0.0000* | ||
| Active | Predicta | 0.1921 | 0.0572^ | |
Table 4: Multiple comparisons of fluoride release among groups by storage periods using dunnett T3.
Discussion and Conclusion
Fluoride release
Fluoride is released from restorative materials in different stages. Water is the first to diffuse into the substance, followed by fluoride ion dissolution and diffusion out of the material [11]. Several variables influence the complicated process of fluoride release from restorative materials. The quantity of fluoride released is affected by intrinsic characteristics such as the material's composition, solubility or porosity [12].
Our findings revealed a statistically significant difference in fluoride release across the three tested materials, with Equia forte HT samples having the greatest fluoride release, followed by active samples, and Predicta samples having the lowest. The F content in these materials is directly related to the increase in the quantity of F released by raising the F concentration gradient between the material and the solution [13].
Furthermore, because the matrix synthesis of GI-based materials is a maturation process, it slowed F release by reducing F diffusion. [14]. Hardness and maturation are increased [15]. It has previously been demonstrated that the curing process, whether light or chemical, influences fluoride release from glass ionomers, resin modified glass ionomers, and dualcured resin materials, and that photo initiated polymerization enhances cross-linking density, resulting in lower resin matrix permeability for fluoride ions [16,17].
However, because Equia Forte ht Fil is a chemically cured material, our findings of increased fluoride emission in non-light cured Equia samples cannot be explained by increased cross-linking due to light curing. Because of the aforementioned problems, Equia may have received the highest F rating. The kind and amount of resin used in RMGICs (active) must be considered, such as UDMA resin, which is a hydrophobic polymer with low water sorption and solubility [ 18,19]. Their hydrophobic nature, supplied by UDMA, results in decreased water absorption and filler dissolution. As simple as it may appear, the level of fluoride ions freed by RMGICs is lower than that of GICs, which might be due to the UDMA changing the acid-base interaction, resulting in less fluoride release [18,19].
The equia specimens emitted fluoride ions in the first 7 days (12.470 -20.010 ppm) and more than discharged by the second period, according to our findings (7.300-17.060).
The substantial amount of fluoride emitted in the first few days is referred to as "The Initial Burst Effect," since Fluoride release from glass ionomer is constrained by concentration and diffusion in the matrix as well as the particles.
Following the initial acid dissolution of powder particle surfaces, a significant amount of fluoride becomes part of the reaction product matrix; this fluoride diffuses rapidly from the matrix exposed on the material's surface and is gradually substituted by fluoride diffusing from the matrix beneath the surface [20-22].
RMGI (activa) shows similar dynamics of fluoride release, An acid-base interaction occurs between carboxylate groups of the acidic monomer and F-containing glass-fillers when resin-based ion leaching materials comprising ionomer-like fluorosilicate glass absorb water, resulting in the release of fluoride ions[23].
As the raw material of the specimen is resinmodified glass ionomer cement, one of the hardening mechanisms of this material is the acid-base reaction; this can be explained by the rapid elution of fluoride liberated as a result of the acid-base reaction, which takes place on the particles glass surface. This was consistent with a number of in vitro investigations that found increased release of fluoride in the first several days than in the last 28 days [18,19,22, 24].
All glass-ionomer systems have an acid/base hardening reaction owing to their glass particles and polyacid components, as well as light and chemical cure capabilities due to their "bioactive ionic resin matrix" component, are all involved in the hardening mechanisms of the Activa resin samples tested in this study [25].
The kind The fluoride release potentiality of resin modified glass is affected by the kind and amount of resin used in the photochemical polymerization technique, as well as the formation of complex fluoride compounds and their interactions [26].
The data showed that the fluoride leakage decreased during the next few days. This was caused by the slower disintegration of glass particles through the pores of the repairs over time. Bulk fluoride release occurs throughout the maturation stage as a result of material interaction with the storage media. This was consistent with other research [18,21,27].
Predicta samples had considerably lower fluoride release than Equia and Activa samples, and there is no significant variation in fluoride release between the two periods. It most likely includes bioactive glass. The breakdown of bio glass rapidly raises the concentration of fluoride ions; nevertheless, the contribution from the dissolution process is extremely little, possibly because to the presence of hydrophobic resins that rejects water.
It is possible that the impact of recharging fluoride is more than the effect of ion release in this material, and that the bioactivity property is greater than the effect of ion release. There is no detailed information available on the composition of predicta bulk bioactive composite, and no study has been conducted. More research is needed to understand the composition and other features of this novel composite.
Fluoride is released from composite resins and RMGIC via the diffusion process [28]. Because the free flow of water is likely hindered in the cured resin matrix, the fluoride source surrounded by a resin matrix may have trouble coming into touch with water. In 1997, Kanchanavasita, Anstice, and Pearson published Kanchanavasita, Anstice, and Pearson [29]. When they evaluated the water sorption qualities of cements, they discovered that GICs and resin-modified glass-ionomer cements absorbed more water than a resin composite. In 1998, Martin and Jodynakiewicz [28,30]. This might explain why resin-modified glass-ionomer cements and glass ionomer cements released more fluoride than fluoridereleasing resin composites, as demonstrated in this work.
Because the release is governed by the mix of filler particles and the matrix of set material, fluoride release after setting continued to follow the same pattern in all three groups in our investigation: Fluoride ions diffuse through the pores of the substance [31]. Previous research on GICs and resin modified GICs found the similar pattern of fluoride emission [18,19,22,32].
Conclusion
In comparison to the predicta bulk bioactive, the active kids exhibited higher fluoride ions release concentrations. The CGI, on the other hand, showed larger fluoride ion release concentrations than the other two materials.
References
- Knibbs PJ. Glass ionomer cement: 10 years of clinical use. J Oral Rehabil 1988; 15:103-15.
- Charlton DG, Murchison DF, Moore BK. Repairability of type II glass polyalkenoate (ionomer) cements. J Dent 1991; 19:249-254.
- Walls AWG. Glass polyalkenoate (Glass–ionomer) cements: A review. J Dent 1986; 14:231-246.
- Craig RG. Restorative dental materials, St. Louis, 1997.
- Smith DC. Composition and characteristics of glass ionomer cements. J Am Dent Assoc 1990; 120:20-22.
- Anusavice KJ. Phillips’ science of dental materials. Philadelphia. USA, 1996.
- Silva RC, Zuanon AC. Surface roughness of glass ionomer cements indicated for atraumatic restorative treatment (ART). Bra Dent J 2006; 17:106-109.
- Najeeb S, Khurshid Z, Zafar MS, et al. Modifications in glass ionomer cements: Nano-sized fillers and bioactive nanoceramics. Int j Mol Sci 2016; 17:1134.
- Šalinović I, Stunja M, Schauperl Z, et al. Mechanical properties of high viscosity glass ionomer and glass hybrid restorative materials. Acta Stomatol Croatica 2019; 53:125.
- Croll TP, Nicholson JW. Glass ionomer cements in pediatric dentistry: Review of the literature. Pediatr Dent 2002; 24:423-429.
- Nigam AG, Jaiswal J, Murthy R, et al. Estimation of fluoride release from various dental materials in different media—An In Vitro study. Int J Clin Pediatr Dent 2009; 2:1.
- Tiwari S, Kenchappa M, Bhayya D, et al. Antibacterial activity and fluoride release of glass-ionomer cement, compomer and zirconia reinforced glass-ionomer cement. J Clin Diagn Res 2016; 10:ZC90.
- Davis HB, Gwinner F, Mitchell JC, et al. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent Mater 2014; 30:1187-94.
- Bueno LS, Silva RM, Magalhães AP, et al. Positive correlation between fluoride release and acid erosion of restorative glass-ionomer cements. Dent Mater 2019; 35:135-43.
- Ruengrungsom C, Burrow MF, Parashos P, et al. Evaluation of F, Ca, and P release and microhardness of eleven ion-leaching restorative materials and the recharge efficacy using a new Ca/P containing fluoride varnish. J Dent 2020; 102:103474.
- Yoda A, Nikaido T, Ikeda M, et al. Effect of curing method and storage condition on fluoride ion release from a fluoride-releasing resin cement. Dent Mater J 2006; 25:261-266.
- Shimura R, Nikaido T, Yamauti M, et al. Influence ofcuring method and storage condition on microhardness of dualcure resin cements. Dent Mater J 2005; 24:70-75.
- 8Abdulla HA, Majeed MA. Assessment of bioactive resin-modified glass ionomer restorative as a new CAD/CAM material. Part I: Marginal fitness study. Indian J Forensic Med Toxicol 2020; 14:865.
- Altamimi A, Majeed MA. Development and assessment of new bioactive glass fiber post. Part II: Ion’s release. Indian J Forensic Med Toxicol 2021; 15.
- Temin SC, Csuros Z. Long-term fluoride release from a composite restorative. Dent Mater 1988; 4:184-6.
- Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, et al. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent Mater J 2013; 32:296-304.
- Salman RF. Fluoride ions release study of different GIF materials. J Bagh College Dent 2006; 18:26-29.
- Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Materials 2007; 23:343-62.
- Cardoso AM, de Sousa Leitão A, Neto JC, et al. Evaluation of fluoride release, pH and microhardness of glass ionomer cements. Pesquisa Brasileira Odont Clín Integrada 2015; 15.
- Yap AU, Tham SY, Zhu LY, et al. Short-term fluoride release from various aesthetic restorative materials. Oper Dent 2002; 27:259-65.
- Kucukyilmaz E, Savas S, Kavrik F, et al. Fluoride release/recharging ability and bond strength of glass ionomer cements to sound and caries affected dentin. Niger J Clin Pract 2017; 20:226–234.
- Attar N, Turgut MD. Fluoride release and uptake capacities of fluoride-releasing restorative materials. Oper Dent 2003; 28:395-402.
- Kanchanavasita W, Anstice HM, Pearson GJ. Water sorption characteristics of resin-modified glass-ionomer cements. Biomaterials 1997; 18:343-349.
- Hsu CY, Donly KJ, Drake DR, et al. Effects of aged fluoride-containing restorative materials on recurrent root caries. J Dent Res 1998; 77:418-25.
- Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Dent Res J 2009; 6:75.
- Ležaja-Zebić M, Jakovljević N, Miletić V. Fluoride release from conventional, resin-modified and hybrid glass ionomer cements. Stomatološki glasnik Srbije. 2018; 65:187-94.
- Kandil M, Sherief D. Marginal adaptation, compressive strength, water sorption, solubility and ion release of a claimed bioactive restorative material. Egyptian Dent J 2021; 67:547-561.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Shukran Abdalhasan Mohamad Samir* and Baydaa Hussein
Department of Pedodontics and Preventive Dentistry, College of Dentistry, University of Baghdad, IraqCitation: Shukran Abdalhasan Mohamad Samir, Baydaa Hussein, Fluoride Release and Solubility of New Bioactive Restoratives used in Pediatric Dentistry Part 1 (Fluoride Release), J Res Med Dent Sci, 2022, 10 (3):168-174.
Received: 04-Feb-2022, Manuscript No. JRMDS-22-51572; , Pre QC No. JRMDS-22-51572 (PQ); Editor assigned: 07-Feb-2022, Pre QC No. JRMDS-22-51572 (PQ); Reviewed: 21-Feb-2022, QC No. JRMDS-22-51572; Revised: 25-Feb-2022, Manuscript No. JRMDS-22-51572 (R); Published: 04-Mar-2022
