Research - (2023) Volume 11, Issue 7
Formulation and Evaluation of the Risperidone Solid Dispersion Using Different Carriers
Zahraa Kareem Hussein1,2* and Khalid Kadhem Al-Kinani1
*Correspondence: Zahraa Kareem Hussein, Department of Pharmaceutics, College of Pharmacy, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Solid Dispersion (SD) is used in improving drugs’ physicochemical properties including solubility and dissolution and it is considered as a smart method of pharmaceutical technology. The drug risperidone (RIS) is prescribed for the short-term management of acute manic or mixed episodes linked to bipolar disorder, both positive and negative signs, and symptoms of schizophrenia. RIS is class II drug as stated by the Biopharmaceutical Classification System (BCS) which means it has low solubility. In this study, attempts were made to enhance dissolution rate and solubility of RIS by solid- dispersion system. Twenty eight formulas of RIS were prepared as a solid- dispersion using different carriers includes poloxamer 407 (PXM407), polyethylene glycol 6000 (PEG6000), Polyvinylpyrrolidone (PVP K30), and poloxamer188 (PXM188) at different drug: carrier ratios (1:1, 1:2, 1:3, and 1:5) by using two different preparation methods (fusion method and solvent evaporation method). The prepared formulas were characterized for drug content, production yield, solubility study, dissolution study, FTIR, DSC, and PXRD .The results indicate that the used carriers show enhancement in drug solubility in the following rank order: PVP K30>PEG 6000> PXM407> PXM188. The best drug: polymer ratio was 1:5 while best preparation method was solvent evaporation. The optimum formula (drug: PVP K30 at ratio of 1:5) is prepared by solvent evaporation method shows 25-fold enhancement in solubility related to pure RIS. Further characterization of the optimum formula shows amorphousization of RIS. It can be concluded that the solid dispersion can under the selected criteria here can be followed to solve the problem of RIS solubility which could possibly result in better RIS bioavailability.
Keywords
Risperidone, Solid dispersion, Solubility, PVP K30, Solvent evaporation, Fusion method
Introduction
Risperidone (RIS) is atypical antipsychotic drug prescribed for the short-term management of acute manic or mixed episodes linked to bipolar disorder where it is administered orally. It is also used to treat schizophrenia and other psychoses. Both the positive and negative signs and symptoms of schizophrenia can be effectively treated with RIS. Behavioral and psychological symptoms of dementia and irritability associated with autism and other psychoses can also be managed with RIS [1]. RIS chemical name is 3- (2- [4-(6- Fluoro- 1,2-benzisoxazol-3-yl)-1- piperidinyl]ethyl)-6,7,8,9- tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one and figure 1 shows the chemical structure of the drug [2]. RIS is an antagonist to dopamine D2, serotonin (5- HT2), adrenergic receptors (1 and 2), and histamine (H1 receptors).
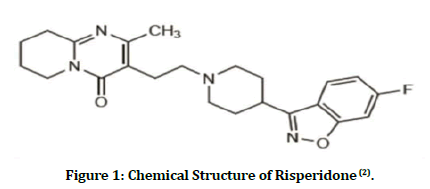
Figure 1: Chemical Structure of Risperidone (2).
As per the Biopharmaceutical Classification System (BCS), RIS is drug of class II meaning it has low aqueous solubility. Therefore, it is necessary to produce an appropriate dosage form that would result in greater solubility and better bioavailability. According to the United States Pharmacopeia, RIS is a white or almost white crystal powder. Practically, insoluble in water, sparingly soluble in alcohol, soluble in dichloromethane.
Its molecular formula C23H27FN4O2, molecular weight 410.5 g/mol, melting Point 170°C, pKa 8.76 and log P 3.27.
Many studies have been done to improve RIS water solubility through different formulation technologies to achieve optimum bioavailability with minimal adverse effects [3-6]. The technologies of Solid Dispersions (SD) are used to improve oral absorption and bioavailability of BSC class II and IV drugs. In solid dispersions the hydrophobic drugs are dispersed in the matrix which is hydrophilic matrix to form SD. There are many polymers that can be used in the preparation of solid dispersion such as Poly Ethylene Glycol (PEG) and Poly Vinyl Pyrolidone (PVP). Also surfactant such as poloxamer 188 and poloxamer 407 are used in the preparation of SD to increase the solubility and then the dissolution of the drugs [7].
Many methods have been used in the manufacturing of SD such as fusion method and solvent evaporation method .The solvent evaporation method is one of the most usually used methods for enhancing solubility of poorly water-soluble drugs in the pharmaceutical industry. Since medication and carrier are combined by a solvent rather than heat as in the melting method, this approach was primarily designed for heat unstable components. Consequently, this technique permits the employment of carriers with abnormally high melting points [8]. The drug and carrier are thoroughly mixed after being solubilized in a solvent which is volatile and subsequently evaporate the solvent at low temperatures, thereby decreasing the risk of drug and carrier thermal breakdown [9]. There are several drawbacks to this method, such as high cost, difficult to select an appropriate solvent for each drug and carrier, and complete solvent evaporation from prepared SD can take long time [10]. In fusion method heating is used to melt a physical mixture of medication and polymer, which is cooled, then solidified while being vigorously stirred. To attain the necessary particle size, the resulting solid form is next smashed, pulverized, and sieved. Although this method has many benefits, such as its low cost and simplicity, it has several drawbacks such as degradation of heat-labile products, being not suitable with for high melting point carriers, and drug and carrier should be compatible to avoid phase separation [11].
The Objective of the Study
The aim of the study was to formulate solid dispersion of RIS using PVP K30, PEG 6000, PXM 188 and PXM407 and study their effect on the solubility and dissolution rate of RIS.
Materials and Methods
Materials
Risperidone, poloxamer 188, poloxamer 407, PEG6000 and PVPK30 were obtained from Hyper Chem, China. Methanol was obtained from GCC-(UK). All of other reagents were of analytical grade.
Preparation of Risperidone Solid Dispersion
Solvent Evaporation Method
Various carriers PXM 407, PEG 6000, PVP K30, and PXM 188 were used in varied ratios (1:1, 1:2, 1:3, and 1:5) representing the drug: carrier ratio (w:w) as shown in table 1 to create the RIS solid dispersion. The carriers and drug were accurately weight. By using a magnetic stirrer, the carriers were individually dissolved in 10 ml of methanol until a clear solution is formed. RIS was then added, and mixing was maintained. Solvent was evaporated at room temperature for 24 hours. The resulting dry material was ground and sieved via a 250-micron sieve then kept in a desiccator for characterization [12, 13].
| Formula Code | Carrier | Drug: Carrier ratio (W:W) | Method of Preparation |
|---|---|---|---|
| SD1 | PEG6000 | 01:01 | Solvent evaporation |
| SD2 | PXM188 | 01:01 | Solvent evaporation |
| SD3 | PXM407 | 01:01 | Solvent evaporation |
| SD4 | PVP K30 | 01:01 | Solvent evaporation |
| SD5 | PEG6000 | 01:02 | Solvent evaporation |
| SD6 | PXM188 | 01:02 | Solvent evaporation |
| SD7 | PXM407 | 01:02 | Solvent evaporation |
| SD8 | PVP K30 | 01:02 | Solvent evaporation |
| SD9 | PEG6000 | 01:03 | Solvent evaporation |
| SD10 | PXM188 | 01:03 | Solvent evaporation |
| SD11 | PXM407 | 01:03 | Solvent evaporation |
| SD12 | PVP K30 | 01:03 | Solvent evaporation |
| SD13 | PEG6000 | 01:05 | Solvent evaporation |
| SD14 | PXM188 | 01:05 | Solvent evaporation |
| SD15 | PXM407 | 01:05 | Solvent evaporation |
| SD16 | PVP K30 | 01:05 | Solvent evaporation |
| SD17 | PEG6000 | 01:01 | Fusion |
| SD18 | PXM188 | 01:01 | Fusion |
| SD19 | PXM407 | 01:01 | Fusion |
| SD20 | PVP K30 | 01:01 | Fusion |
| SD21 | PEG6000 | 01:02 | Fusion |
| SD22 | PXM188 | 01:02 | Fusion |
| SD23 | PXM407 | 01:02 | Fusion |
| SD24 | PVP K30 | 01:02 | Fusion |
| SD25 | PEG6000 | 01:03 | Fusion |
| SD26 | PXM188 | 01:03 | Fusion |
| SD27 | PXM407 | 01:03 | Fusion |
| SD28 | PVP K30 | 01:03 | Fusion |
Table (1): Composition of RIS Solid Dispersion Formulas and Method of Preparation.
Fusion Method
The fusion process involves liquefying a predetermined number of carriers and drug in varied ratios (1:1, 1:2, 1:3, and 1:5) representing the drug: carrier ratio (w: w) as shown in table 1. First, the carrier was heated in a porcelain Petri-dish just above its melting point then drug was added and the mixture was constantly stirred for five minutes before being chilled in an ice bath. After that, the dried material was ground and sieved via a 250-micron sieve then stored in a desiccator for characterizations. A modification of the above method was used for PVP K30, namely closed melting point method. This method includes the addition of water to the carrier and heated, the drug dispersed in the heated mixture with constant stirring then cooled it. The dried mass was crushed and ground in mortar and pestle and passed through 250-micron sieve and stored in a desiccator.
Preparation of Physical Mixture
In the exact ratios as the optimum SD formula, the drug and polymer were uniformly mixed by using mortar and pestle for 10 minutes to prepare the physical mixture [14].
Characterization of Prepared Risperidone Solid Dispersion Formulas
Percent Yield (PY %) Determination of Prepared RIS Solid-Dispersion.
Percent yield (PY %) of the prepared RIS-solid dispersion was calculated to determine the efficiency of each of SD preparation method for each formula. The PY% was set by calculating the ratio of the actual weight of the resulting solid dispersion on the theoretical mass of drug and carrier [15].

Determination of Risperidone Content in Prepared Solid Dispersion
A volume of 100 mL of 0.1N HCl was used to dissolve the RIS solid dispersion corresponding to 10 mg of RIS, which was then mixed using a vortex mixer. Serial dilutions of the solution were performed before employing Whatman filter paper to filter it. Spectrophotometric analysis estimated the RIS content at 277 nm. The following equation was used to calculate the percentage of drug content in the produced solid dispersion [16].

Determination of Saturated Solubility of Risperidone Solid -Dispersion
In aqueous solutions, the solubility of the produced RIS solid dispersion was evaluated. The solubility study was done by adding excess amounts of RIS solid- dispersion into screw capped vials holding 10 ml of water. The tightly sealed vials are kept in water bath shaker at 25 ± 0.5 °C for 48 hours. After centrifuging and filtering the saturated solutions were filtered through a 0.45μm membrane filter, then the amount RIS dissolved was determined using a UV spectrophotometer [17, 18].
In-Vitro Dissolution Studies of RIS-Solid Dispersion and Pure Risperidone
The dissolution profiles of pure RIS and the RIS from the prepared solid dispersion formulas were studied. The test is performed in 900 ml phosphate buffer of pH 6.8, rotation was at 50 rpm and the media temperature was kept at 37± 0.5 °C during the test using USP standard dissolution apparatus II with paddle. Five ml aliquot samples were taken, and replaced with fresh phosphate buffer pH 6.8 solution of the same volume at time points of 5, 10, 15, 20, 30, 40, 50, and 60 minutes. Then, after filtering the samples, spectrophotometric measurements are made to determine percentage drug released.
Factors Affecting Dissolution Behavior of RIS from Solid Dispersion
The impact of different polymer type (PEG6000, PXM188, PXM407, and PVP K30) on the drug release of prepared solid dispersion was studies through formulas SD13, SD14, SD15, and SD16, respectively. These formulas had the same ratio of drug: polymer (1:5) and same method of preparation which is solvent evaporation method. The results are compared to the release of pure drug.
The influence of different drug: carrier ratio on the drug release from solid dispersion is also studied through formulas SD4 (1:1), SD8 (1:2), SD12 (1:3) and SD16 (1:5). All of these formulas were prepared by solvent evaporation method and the carrier used was PVP K30.
Release Kinetic Analysis of Risperidone Solid- Dispersion Formulas
Release kinetics of RIS solid dispersion formulas (SD16, SD8, SD12, SD13, SD15, SD4, SD25, SD6, SD7 and SD10) was determined using DD solver software. The in-vitro release numbers were fitted into the kinetic model to examine the mechanism of drug release. The models used were Higuchi, first order and first order with Fmax release models. The model with the maximum correlation coefficient (R2) is considered to be the best fitted model. The DD Solver program used to obtain release kinetic model of RIS solid dispersion formulas.
Evaluations of the Optimum Formula
Fourier-Transform Infrared Spectroscopy (FTIR)
The FTIR study of RIS, PVP K30 polymer, the physical mixture of RIS and PVP K30 carrier and the optimum formula (SD16) is conducted to look at the intermolecular interactions among the drug and polymers. The samples were condensed with potassium bromide (KBr) as a disc, and read by FTIR apparatus (Shimadzu 8000, Japan). The scanning range was 4000- 400 cm-1 [19].
Differential Scanning Calorimeter (DSC)
Thermal characteristics of the pure RIS, PVP K30 polymer and optimum formula (SD16) were determined by a programmed thermal analyzer device (Shimadzu, DSC- 60, and Japan). Approximately 3 mg sample of each was placed in none hermetically aluminum pan and heated at rate 10°C per min up to 300°C. The analysis was carried out under atmosphere flow conditions [20, 21].
Powder X-Ray Diffraction (PXRD)
Powder X-ray was used to analyse the crystallinity of the pure RIS, the physical mixture of RIS and PVP K30 and selected formula SD16. The measurements were made under these circumstances: the filter K, the target metals Cu, 45kV voltage, and 30mA current. Samples scanned over a 2 θ range of 3 -80C° the step size was 0.04° [22, 23].
Statistical Analysis
The results of the experiments are expressed as a mean triplicate (sample ± standard deviation) and the analysis was done with the aid of one-way analysis of variance (ANOVA) and independent sample T test using IBM SPSS Statistic version 26 software upon which the results would be significant when p < 0.05, and the results would be non-significant if p> 0.05.The DD Solver program also used to obtain release kinetic model of RIS solid dispersion formulas [24].Results and Discussion
Determination of Saturation Solubility of Risperidone as a Pure Drug
The solubility studies were done in distilled water (D.W.), 0.1N HCl and phosphate buffer at pH 6.8 at 25 °C for 48 hour. Risperidone is a weak base and its pKa value is 8.76 and as expected showed a higher solubility in an acidic medium to about 10.5 mg/ml. In contrast, risperidone solubility in phosphate buffer was low with a solubility value of 1.52 mg/ml. On the other hand, the solubility of risperidone in water was the lowest of about 0.063mg/ml which is considered practically insoluble as mentioned in the literature [25].
Characterization of Risperidone Solid Dispersion
The prepared RIS-solid dispersions using different formulas and methods as described in the materials and methods are characterized as in the following subsections:
Percent Yield (PY %) of Prepared RIS-Solid Dispersion
The PY% of the prepared RIS-solid dispersion was determined to choose the best preparation method of solid dispersion. Both solvent evaporation and fusion methods gave acceptable PY% ranged from (90-99.9 %). All the formulations show high yield percentage, which indicates the reproducibility of fusion method and solvent evaporation method techniques for preparation of RIS solid- dispersion and its efficiency [26].
Drug Content of the Prepared Risperidone Solid Dispersion Formulas
All prepare formulations showed satisfactory drug content ranging between 90- 102% (W/W). This indicating that there was no loss and uniform distribution of RIS during the preparation process [27].
Saturated Solubility of Risperidone Solid- Dispersion
The saturated solubility of RIS solid-dispersion performed in distilled water maintained at 25°C temperature for 48 hour the results was within fluctuated range from (0.178-1.62) mg/ml.
Effect of Preparation Method on the Saturated Solubility of Risperidone
There was significant difference (p<0.05) between solvent evaporation and fusion method in the preparation of RIS as a solid dispersion in terms of solubility figure 2. This difference may be attributed to the that solventbased methods generates solid dispersion with smaller particle size and comparatively larger surface area, while, a fusion-based method usually results in a low-porosity material owing to the decrease in free space existing in the carrier-drug mixtures [28]. Hence, the shape and the size of the final particle products produced by fusion method are typically unrelated to the preparing method; however they are dependent on the grinding process.
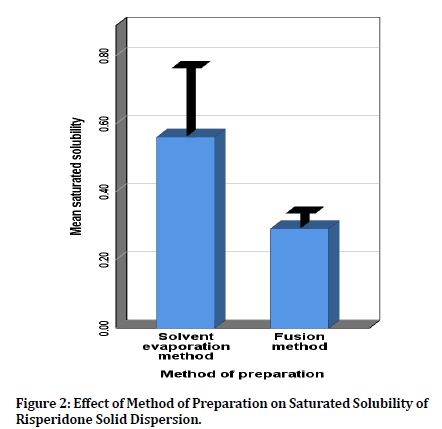
Figure 2: Effect of Method of Preparation on Saturated Solubility of Risperidone Solid Dispersion.
Effect of Carrier Type on the Saturated Solubility of Risperidone
The mean saturated solubility of RIS solid dispersion using different carriers, (RIS: PEG6000 1:5), (RIS: PMX188 1:5), (RIS: PMX407 1:5) and (RIS: PVPK30 1:5), is shown in figure 3. As it is obvious all types of carrier resulted in an increase in the saturated solubility of RIS compared to that of pure drug, but PVP K30 resulted in the highest (p<0.05) increase in solubility compared to other used carriers. This highest increase in solubility could be due to that PVP K30 inhibits crystal formation of drug, increase the wettability and surface area of the drug, and subsequent creation of amorphous soliddispersion to enhance solubility of RIS [29].
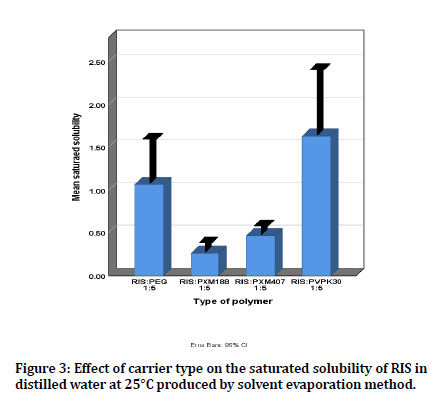
Figure 3: Effect of carrier type on the saturated solubility of RIS in distilled water at 25°C produced by solvent evaporation method.
Effect of RIS: PVP K30 ratio on the saturated solubility of risperidone
As shown in figure 4, RIS saturated solubility increased significantly (p<0.05) with increasing the PVP K30 content in the rank order (1:1 ˂ 1:3 ˂ 1:5) ratios of formulas prepared by the solvent evaporation method. Solubility improvement may be attributed to the shared performance of the surface activity, solubilization, and wetting effect of PVP K30 [30]. The water soluble amorphous carrier PVP K30 displays high solubility when dispersed with poorly water soluble drugs and the extent of solubility be determined by the molecular weight and the concentration of PVP. Thus, PVP has been prepared to be the greatest carrier which may be owing to its larger solubility profile and hydrogen bonding with the drug as stated in several studies [31].
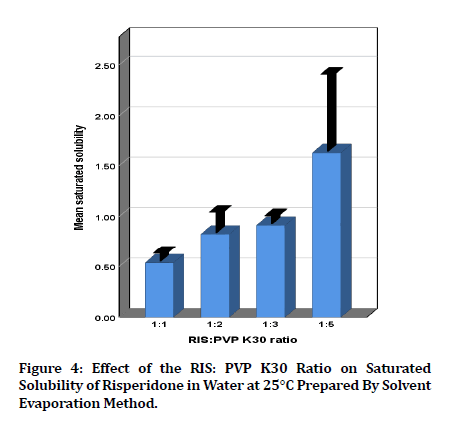
Figure 4: Effect of the RIS: PVP K30 Ratio on Saturated Solubility of Risperidone in Water at 25°C Prepared By Solvent Evaporation Method.
In-vitro Release of Risperidone Solid Dispersion:
Effect of Polymer Type
At a constant ratio of drug to polymer (1:5), PVP K30 (SD16) showed a complete release of the drug in just 5 minutes in comparison with other type carriers and pure RIS, where the release was of 86%, 70%, 63% and 7% for PEG6000 (SD13), PMX188 (SD14), PXM407 (SD15) and pure RIS, respectively. Release profiles are shown in Figure 5. This noticeable enhancement (p˂0.05) in RIS release from PVP K30 solid dispersion can be credited to the greater solubility of RIS solid-dispersion, which is owing to better solubility of RIS in the presence of PVP K30. Besides, PVP K30 as hydrophilic polymer could increase the wettability of drug and therefore expands the dissolution rate of RIS SD [32].
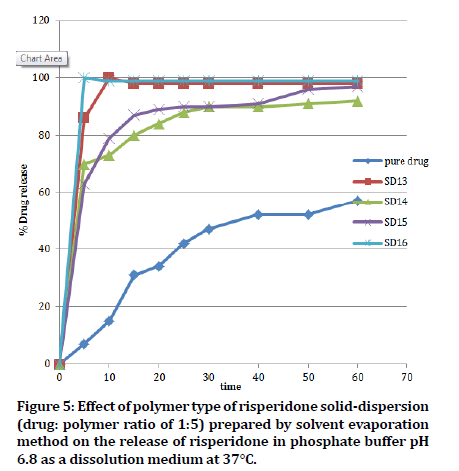
Figure 5: Effect of polymer type of risperidone solid-dispersion (drug: polymer ratio of 1:5) prepared by solvent evaporation method on the release of risperidone in phosphate buffer pH 6.8 as a dissolution medium at 37°C.
Effect of RIS: Carrier ratio
The influence of the different RIS: Carrier ratio on the dissolution behavior of RIS solid dispersion is shown in Figure 6.The Figure represented the release profiles of the RIS from its solid-dispersion using PVP K30 as a carrier and solvent evaporation method as the preparation method, in addition to the release of naked RIS. It was observed that when the amount of PVP K30 was increased in formulas (SD16 > SD12> SD8> SD4), the drug release from solid dispersion increased significantly (P<0.05). The formula SD16 exhibited the highest percent of RIS release by (100%) after 5 minutes as compared to SD12 (87%), SD8 (72%), SD4 (71%) and pure RIS (7%). The enhancement in in vitro dissolution of RIS solid dispersions that are formulated with higher ratios of the water-soluble carriers is owing to the improved drug wettability and conversion to the amorphous forms, which are confirmed by DSC and PXRD, shown later in this work, and the high probability of a reduction in particle size. This could be as a result of the hydrophilic property of PVP K3 and superficial adsorption of drug particles on this carrier in a very fine state of subdivision or molecular form. The resultant reduction in particle size and the rise in the interfacial area of connection between the drug particles and the solvent augmented the solubility of the drug compared to the naked drug [33].
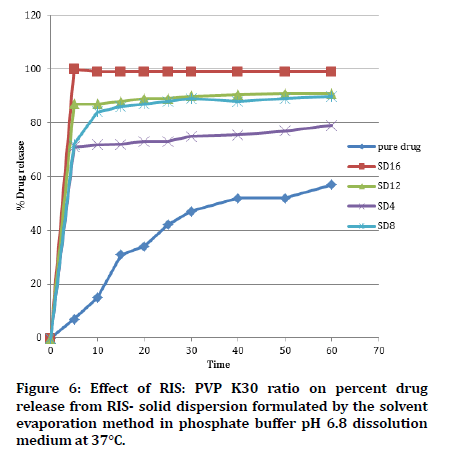
Figure 6: Effect of RIS: PVP K30 ratio on percent drug release from RIS- solid dispersion formulated by the solvent evaporation method in phosphate buffer pH 6.8 dissolution medium at 37°C.
Release Kinetics
After fitting the in vitro release data into various kinetic models, the regression parameters obtained are shown in table 2. The best fit for the various models examined for the solid- dispersions are graded in the order of first order with Fmax > first order> Higuchi . It was detected that the release data was set up to fit greatest into the first order with Fmax model as its correlation coefficient values (R2) were observed to dominate over the (R2) values of the other models.
| Mathematical models of drug release | |||||||
|---|---|---|---|---|---|---|---|
| Formula code | Higuchi model | First order model | First order with Fmax | ||||
| kH | R square | k1 | R square | k1 | Fmax | R square | |
| SD16 | 17.62 | -0.045 | 7.463 | 0.9991 | 4.343 | 99.111 | 0.9999 |
| SD4 | 13.338 | 0.1642 | 0.089 | 0.3088 | 0.591 | 74.61 | 0.9912 |
| SD12 | 15.95 | 0.0654 | 0.344 | 0.8826 | 0.707 | 89.465 | 0.998 |
| SD13 | 17.353 | 0.0871 | 0.399 | 0.9964 | 0.427 | 98.362 | 0.9988 |
| SD15 | 15.978 | 0.5156 | 0.158 | 0.9503 | 0.213 | 92.213 | 0.9917 |
| SD8 | 15.524 | 0.2489 | 0.197 | 0.8693 | 0.332 | 88.167 | 0.9986 |
| SD25 | 14.584 | 0.496 | 0.11 | 0.8436 | 0.195 | 85.022 | 0.9992 |
| SD6 | 16.494 | 0.296 | 0.243 | 0.96 | 0.318 | 93.64 | 0.9936 |
| SD7 | 12.57 | 0.4262 | 0.061 | 0.4442 | 0.231 | 72.419 | 0.9972 |
| SD10 | 17.216 | 0.2746 | 0.328 | 0.9829 | 0.375 | 97.047 | 0.9901 |
Table (2): Release Kinetics Parameters of Risperidone Solid Dispersion Formulas.
Selection of the Optimum Formula
Selection of the optimum formula was made based on the solubility reading and the dissolution profile of RIS solid dispersion. The optimum formula was SD16, which is composed of RIS-PVP K30 solid dispersion at a ratio 1:5 and prepared by solvent evaporation method. It is characterized by enhanced solubility and better percent release of RIS. Therefore it was subjected to further invitro evaluation studies.
Evaluation of Optimum Formula
Fourier Transforms Infrared Spectroscopy (FTIR)
The FTIR spectrum of the pure RIS Figure 7-A showed characteristic peaks at 3064 cm-1 due to stretching vibration of (C-H) of aromatic ring and peaks at 2941cm- 1, 2758cm-1 due to stretching vibration of (C-H) alkane (asymmetric and symmetric). Also peak at 1644 cm-1 is due to stretching vibration of carbonyl group (C=O). Peak at 1531 cm-1 is due to stretching vibration of (C=N). Peaks at 1456 cm-1 due to stretching vibration of (C=C) of aromatic ring. Peaks at 1406, 1353, 1302 cm-1 due to bending of (C-H) alkane. Peak at 1268 cm-1 due to stretching vibration of (C-F) aromatic ring. Peak at 1240cm-1 is due to stretching vibration of (=C-O). Peak at 1186 cm-1 is due to stretching vibration of (CN). Peaks at 1129, 1086, 1023 cm-1 are due to stretching vibration at (C-N-C). Peaks at 962, 928, 898 cm-1 are due to disubstitution of benzene ring [34].
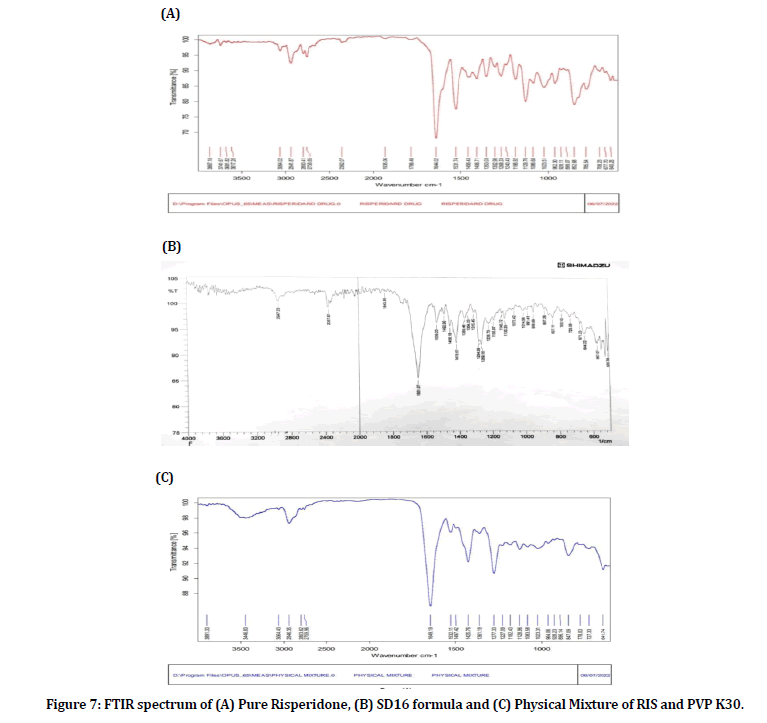
Figure 7: FTIR spectrum of (A) Pure Risperidone, (B) SD16 formula and (C) Physical Mixture of RIS and PVP K30.
The spectra of selected SD16 formula showed all the characteristic peaks of RIS, demonstrating that there was no chemical interaction between RIS and PVP K30. The decreased intensity of RIS peaks in the SD spectrum (panel B) compared to the naked drug spectrum (panel A) may be due to dilution effect of the polymer.
Differential Scanning Calorimeter (DSC)
The DSC thermograms of pure drug RIS, PVP K30 polymer, and SD16 formula are shown in figure 8. The DSC thermograms of RIS displayed a sharp endothermic peak at 172.39°C which represents the melting point of RIS which revealed the crystallinity nature of RIS as reported in literature (3, 4). DSC thermograms of PVP K30 showed broad endothermic peak at 88.13°C which may be due to residual moisture and the glass transition temperature (Tg) of PVP K30 appeared at 160°C. DSC thermograms of SD16 showed that there was a single endothermic peak (156.37°C) which was around the PVP K30 glass transition temperature (Tg). While the unclear presence of RIS endothermic peak at (172.39°C) might be attributed to the existence of RIS in an amorphous state rather than its original crystalline state.
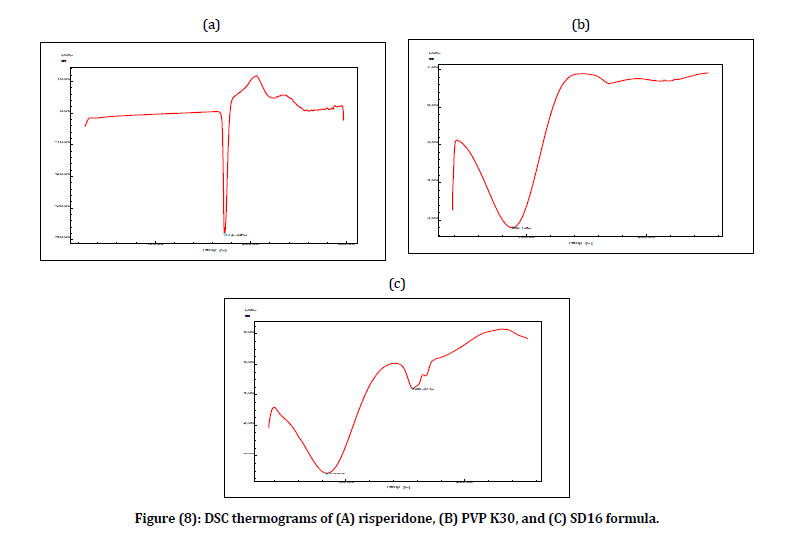
Figure 8: DSC thermograms of (A) risperidone, (B) PVP K30, and (C) SD16 formula.
Powder X-Ray Diffraction (PXRD)
With the aim of find out the crystallinity of pure RIS, physical mixture of RIS and PVP K30 polymer and optimum formula (SD16), powder X-ray diffraction was used. The XRD studies were pursued to gain insight into the physical environment of the drug in the solid dispersion formulation. Figure 9 and 10 show the XRD spectra of pure RIS and the SD16 formula, respectively. The XRD pattern of RIS showed the great peak of high intensity at 2 Theta 20.9872 and five other less prominent peaks at 2 theta 14.0024, 18.6207, 19.4645, 22.8554 and 28.6129 with intensity of 3518,853, 1654,851, 1035 and 1100, respectively because of the native crystalline arrangement of RIS. However, there were a disappearance and reduction in these peaks intensities for the SD16 formula sample. The loss of RIS peak in the SD16 proves the presence of RIS in amorphous form. The important disappearance and/or decrease in the intensity of peaks was detected in SD16 PXRD arrangement was an element of increasing drug solubility and dissolution.

Figure 9: Powder XRD of naked risperidone.

Figure 10: Powder XRD of the optimum (SD16) formula.
Conclusion
Based on the results gained from the current study, it can be established that the poor solubility of RIS (BCS II drug) was effectively improved via solid- dispersion technique using different carriers like PVP K30, PEG 6000, PXM 407 and PXM 188. However, the carrier PVP K30 with (1:5) ratio seemed to be the best carrier in comparison with other carriers. The formula (SD16) with RIS: PVP K30 (1:5) ratio seemed to be the optimum formula which was prepared by solvent evaporation method for improving the solubility and dissolution rate of RIS. The FTIR spectra showed no specific RIS-PVP K30 interactions. The DSC thermograms and X-ray diffraction showed the conversion of the RIS powder from the crystalline form to the amorphous form by solid dispersion which caused the improved the solubility of RIS.
Acknowledgement
The authors would like to thank the Collage of pharmacy, University of Baghdad and specifically the faculty and staff of the Department of Pharmaceutics for the assistance and the generous support throughout all the stages of this study.
Conflicts of Interest
None
References
- Rowe RC, Sheskey P, Quinn M. Handbook of pharmaceutical excipients. Libros Digitales-Pharmaceutical Press 2009.
- Brayfield A. Martindale: The complete drug reference. 2017.
- Mohammed KA, Ibrahim HK, Ghorab MM. Effervescent tablet formulation for enhanced patient compliance and the therapeutic effect of risperidone. Drug Deliv 2016; 23:297-306.
- Kazi M, Al-Qarni H, Alanazi FK. Development of oral solid self-emulsifying lipid formulations of risperidone with improved in vitro dissolution and digestion. Eur J Pharm Biopharm 2017; 114:239-49.
- Farid M, Jaffer M. Formulation and in vivo evaluation of risperidone spherical agglomerates prepared for earlier absorption of risperidone. World J Pharm Res 2019; 8:13.
- Sbârcea L, Tănase IM, Ledeți A, et al. Encapsulation of risperidone by methylated β-cyclodextrins: physicochemical and molecular modeling studies. Molecules 2020; 25:5694.
- Ismail MY, Ghareeb MM. Enhancement of the solubility and dissolution rate of rebamipide by using solid dispersion technique (Part I). Iraqi J Pharm Sci 2018; 55-65.
- Tran P, Pyo YC, Kim DH, et al. Overview of the manufacturing methods of solid dispersion technology for improving the solubility of poorly water-soluble drugs and application to anticancer drugs. Pharmaceutics 2019; 11:132.
- Cid AG, Simonazzi A, Palma SD, et al. Solid dispersion technology as a strategy to improve the bioavailability of poorly soluble drugs. Ther Deliv 2019; 10:363-82.
- Baghel S, Cathcart H, O'Reilly NJ. Polymeric amorphous solid dispersions: a review of amorphization, crystallization, stabilization, solid-state characterization, and aqueous solubilization of biopharmaceutical classification system class II drugs. J Pharm Sci 2016; 105:2527-44.
- Bhaskar R, Monika OL, Ghongade RM. Solid dispersion technique for enhancement of solubility of poorly soluble drug. Indian J Pharm Boil Res 2018; 6:43-52.
- Rahman Z, Zidan AS, Khan MA. Risperidone solid dispersion for orally disintegrating tablet: Its formulation design and non-destructive methods of evaluation. Int J Pharm 2010; 400:49-58.
- Taher SS, Sadeq ZA, Al-Kinani KK, et al. Solid lipid nanoparticles as a promising approach for delivery of anticancer agents. Mil Med Sci Lett 2022; 91.
- Hyun SM, Lee BJ, Abuzar SM, et al. Preparation, characterization, and evaluation of celecoxib eutectic mixtures with adipic acid/saccharin for improvement of wettability and dissolution rate. Indian J Pharm 2019; 554:61-71.
- Al-Hassani HR, Al-Khedairy EB. Formulation and in-vitro evaluation of meloxicam solid dispersion using natural polymers. Iraqi J Pharm Sci 2021; 30:169-78.
- AlKhalidi MM, Jawad FJ. Enhancement of aqueous solubility and dissolution rate of etoricoxib by solid dispersion technique. Iraqi J Pharm Sci 2020; 29:76-87.
- Soliman MA, Ibrahim HK, Nour SA. Diacerein solid dispersion loaded tablets for minimization of drug adverse effects: statistical design, formulation, in vitro, and in vivo evaluation. Pharm Dev Technol 2021; 26:302-15.
- Alkufi HK, Kassab HJ. Formulation and evaluation of sustained release sumatriptan mucoadhesive intranasal in-situ gel. Iraqi J Pharm Sci 2019; 28:95-104.
- Al-Akayleh F, Adwan S, Khanfar M, et al. A novel eutectic-based transdermal delivery system for risperidone. AAPS PharmSciTech 2021; 22:1-1.
- Krishnamoorthy V, Sen S, Prasad VP. Physicochemical characterization of risperidone solid dispersions. Acta Pharm Sci 2011; 53.
- Jassim ZE, Al-Kinani KK, Alwan ZS. Preparation and evaluation of pharmaceutical cocrystals for solubility enhancement of dextromethorphan HBr. 2021.
- Fitriani L, Fadhila M, Zaini E. Preparation of Efavirenz-PVP K-30 Solid Dispersionby Spray Drying Technique. Res J Pharm Biol Chem Sci 2015; 6:925-30.
- Sbârcea L, Tănase IM, Ledeți A, et al. Risperidone/randomly methylated β-cyclodextrin inclusion complex—compatibility study with pharmaceutical excipients. Molecules 2021; 26:1690.
- Zuo J, Gao Y, Bou-Chacra N, et al. Evaluation of the DDSolver software applications. Biomed Res Int 2014.
- Cao Z, Wang Z, Gao F, et al. Thermodynamic analysis and molecular dynamic simulation of the solubility of risperidone (form I) in the pure and binary solvents. J Mol Liq 2022; 359:119061.
- Arun RR, Elwin J, Jyoti H, et al. Formulation and evaluation of ketoprofen solid dispersion incorporated topical gels. Europ J Biomed and Pharm Sci 2016; 3:156-64.
- Pharmacopeia US. The United States Pharmacopeia, USP 41/The National Formulary. InRockville, MD: US Pharmacopeial Convention 2018.
- Huang S, Williams RO. Effects of the preparation process on the properties of amorphous solid dispersions. AAPS PharmSciTech 2018; 19:1971-84.
- Sui X, Chu Y, Zhang J, et al. The effect of PVP molecular weight on dissolution behavior and physicochemical characterization of glycyrrhetinic acid solid dispersions. Adv Polym Technol 2020; 1-3.
- Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30Res Pharm Sci 2010; 5:49.
- Thalluri C, Amin R, Mandhadi JR, et al. Central composite designed fast dissolving tablets for improved solubility of the loaded drug ondansetron hydrochloride. Biomed Res Int 2022.
- Homayouni A, Sadeghi F, Nokhodchi A, et al. Preparation and characterization of celecoxib solid dispersions; comparison of poloxamer-188 and PVP-K30 as carriers. Iran J Basic Med Sci 2014; 17:322.
- Hussein LS, Al-Khedairy EB. Solubility and dissolution enhancement of ebastine by surface solid dispersion technique. Iraqi J Pharm Sci 2021; 30:122-32.
- Daadoue S, Al-Remawi M, Al-Mawla L, et al. Deep eutectic liquid as transdermal delivery vehicle of Risperidone. J Mol Liq 2022; 345:117347.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Zahraa Kareem Hussein1,2* and Khalid Kadhem Al-Kinani1
1Department of Pharmaceutics, College of Pharmacy, University of Baghdad, Baghdad, Iraq2Wassit Health Directorate, Ministry of Health and Environment, Wassit, Iraq
Citation: Zahraa Kareem Hussein, Khalid Kadhem Al-Kinani, Formulation and Evaluation of the Risperidone Solid Dispersion Using Different Carriers, J Res Med Dent Sci, 2023, 11(7):20-28.
Received: 26-Jun-2023, Manuscript No. jrmds-23-107006; Accepted: 29-Jun-2023, Pre QC No. jrmds-23-107006; Editor assigned: 29-Jun-2023, Pre QC No. jrmds-23-107006; Reviewed: 13-Jul-2023, QC No. jrmds-23-107006; Revised: 18-Jul-2023, Manuscript No. jrmds-23-107006; Published: 25-Jul-2023
