Research Article - (2022) Volume 10, Issue 12
Green Synthesis of Silver Nanoparticles from Hypericum Perforatum and Evaluation of Antifungal Activity
Chandran Satheesh Kumar1*, Srinivasagam Raja Sankar2, Kaliyaperumal3 and Prabu K4
*Correspondence: Dr. Chandran Satheesh Kumar, Department of Anatomy,Bharath Institute of Higher Education and Research, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Melmaruvathur, Chennai, Tamil Nadu, India, Email:
Abstract
Nanoparticles shows improved characteristics because their size, morphology and distribution, and are extensively used in various scientific fields. Among metallic nanoparticles, Silver Nanoparticles (AgNPs) are very significant particularly because of their physicochemical and antimicrobial properties which help in molecular diagnostics, therapies and devices used for medical processes. A major adverse effect of chemical synthesis is that it involves the use of hazardous chemicals and toxic by-products are obtained. Thus, there is a requirement for eco-friendly and economic methods to synthesize them and the utilization of aqueous or alcoholic plant extract are quickly expanding and gaining significance. Various plant extracts are used for the green synthesis of AgNPs, one among them is Hypericum perforatum. Hypericum perforatum aqueous extract was used for the synthesis of AgNPs and its antifungal action was proved. This article explains about their main physical chemical properties of synthesized AgNPs and some of their medical applications.
Keywords
Hypericum perforatum, Silver nanoparticles, Green synthesis, SEM, Antifungal effects
Introduction
In recent times, the Nanoparticles (NP) from noble metals have been the target of several researches regarding their unique optical, chemical, electronic, mechanical and magnetic properties [1]. Their particular effects are distinct because of their size and dimension of their specific surface. They are applied in various fields comprising catalysing few reactions, in the electronic field, in textile industry, in photonics, in photography, in cosmetics industry, technical medical products, in dye industry, in the diagnosis field, in the treatment of various chronic and acute disorders such as cancer, malaria, hepatitis and AIDS and as vectors for some drugs [2]. It is also been reported that plants act as a better platform for NP synthesis as it exhibits greater affinity towards sulphur or phosphorus containing biomolecule present in the plant cells [3].
The biosynthesis of transition metal nanoparticles is gaining significance because of their low toxicity, biocompatibility and environmentally friendly nature. Among nanoparticles, silver nanoparticles have potential applications in the area of life sciences, especially in food chemistry, agriculture and forensic science [4]. Antifungal effect of ionic or nanoparticle silver has a great prospective for use in controlling spore producing fungal plant pathogens. Silver is least toxic to humans and animals when compare with synthetic fungicides. Different modes of action showing a wide range of biological pathways of microbes gives a vital benefit for avoiding the resistance progression, which has been enhancing vital in terms of current issues for the chemical management of many plant fungal diseases. Since the efficiency of silver is greatly influenced by application timing, preventative applications of silver particles and nanoparticles work way better before spores enter and colonize inside the plant tissue [5].
New innovative methods are being practiced because of the escalating demand in the manufacture of green NP to combat physical and chemical methods. The most significant benefit of the green synthesis approach of NPs, it reduces the production of hazardous by-products and it is important to follow fundamental principles of green chemistry to implement these sustainable processes [6]. Green synthesis explores unexploited plant sources in the progression of AgNPs. The major particles move towards the AgNPs green synthesis which is biocompatible, cost effective and environmentally safe. The decrease of silver ions occurs when the silver nitrate solution reacts with aqueous extract of plant extract at 60°C [7].
The probability of finding novel active antifungal compounds for pathogenic fungal species has been discussed these days [8]. Hypericum perforatum extracts were proved for its antifungal action. The action of hypericin mediated photodynamic antimicrobial properties on pathogenic fungi is known. The main active component of H. perforatum is hypericin the concentration of active compounds in the different regions of the plant varies depending on the soil's characteristics and climatic conditions [9]. Hence, considering the advantages of plant extract, the present study was done to examine the synthesis of silver nanoparticles and to assess the antifungal action of silver nanoparticles from Hypericum perforatum plant extracts.
Materials and Methods
Preparation of aqueous extract
Fresh plant materials of Hypericum perforatum were collected from the local market and the aqueous extract of the sample was prepared using the freshly collected leaves (10 g), were washed in running tap water and then in distilled water, boiled in 100 ml of distilled water, at 60°C for 5 minutes. The extract is filtered through gauge cloth and used for further experiments.
Synthesis of silver nanoparticles
The chemical, AgNO3 was purchased from hi media laboratories Pvt. Limited, Mumbai, India and was used for the present study. For the synthesis of silver nanoparticle, 6 ml of the aqueous extract of Hypericum perforatum was added to 94 ml of 1 Mm (10-3 M) solution of silver nitrate in 250 ml Erlenmeyer flask. The reaction was performed in dark (to minimize photo activation of silver nitrate), at room temperature. The suitable controls were maintained throughout the conduct of experiments [10].
UV-visible spectra analysis
The bio-reduction of Ag+ in aqueous solution was monitored by periodic sampling of aliquots (0.2 ml) of the suspension, then diluting the samples with 2 ml of deionized water and subsequently measuring UV-visible spectra, at the wavelength of 200 to 700 nm in Beckman model no. DU 40 spectrophotometer at a resolution of 1 nm [11].
Scanning electron microscope
This study was undertaken to know the size and shape of the silver nanoparticles biosynthesized using an aqueous extract of Hypericum perforatum [12]. After nanoparticle synthesis, the sample was filtered via Millipore filters of 0.2 nm pore size to remove any contaminants interfering with the SEM images. About 25 μl of the sample was pipetted out and loaded on a ‘stub’ provided for SEM analysis. The cathode has a permanent magnet for creating an efficient glow discharge for sputtering. It is probable to set the chamber pressure in addition to the sputtering current. This keeps the uniformity of the coating to be controlled so that a shadow free coating can be attained. The photographs of nanoparticles were obtained in a scanning electron microscope (JEOL, Japan, model 6360, in the department of geology, UOM, guindy). The details concerning magnification used, applied voltage, and size of the contents of the images were fixed on the photographs themselves [11].
Recovery of silver nanoparticles by ultracentrifugation
For characterization of silver nanoparticles formed in the aqueous extract, about 1 litre of 1 mm silver nitrate solution containing 60 ml of aqueous leaf extract of Hypericum perforatum was prepared and incubated in dark. After bio-reduction, the solution consisting of hydrosols of silver nanoparticles and biomolecules from the aqueous extract of Hypericum perforatum was subjected to centrifugation at 10000 rpm for 10 minutes, washed twice and the pellet was air dried. Later the supernatant was subjected to centrifuge at 25900 rpm (75000x g), for 30 minutes. The pellet was dissolved in 0.1 ml of deionized water and air dried. The differential centrifugation helped in getting rid of the larger particles and biomolecules.
XRD measurements
The air dried nanoparticles were coated onto an XRD grid and analysed for the formation of Ag nanoparticles by Philips x-ray diffract meter with generator operated at a voltage of 40 KV and a current of 30 mA with Cu Kα1 radiation. The diffracted intensities were measured from 30˚ to 80˚ 2θ angles [12].
Antifungal activity by well diffusion assay
Silver nanoparticles synthesized using aqueous leaf extract of Hypericum perforatum were tested for their potential antimicrobial activity against few phytopathogens. Fusarium oxysporum, Macrophomina phaseolina and botrytis sphere used as test organisms. The well, diffusion assay was performed with fungal culture inoculated on Potato Dextrose Agar (PDA) plates, about 30 μl of 0.5 mg, 1 mg and 1.5 mg of AgNO3 and silver nanoparticles were loaded into each well and control well was maintained. In another set up 4 different wells were made into a PDA medium. 30 μl of sterile water added into the initial well which acts as a control. 30 μl of plant extract added into 2nd well, 30 μl contains 1 mg concentration of silver nitrate and silver nanoparticles were inoculated into 3rd and 4th well respectively and incubated at room temperature for 3 to 5 days [13].
Poison plate method
Different concentrations of silver nanoparticles (10 mg, 20 mg) were mixed into potato dextrose agar and poured into petri dishes. Fungus such as Fusarium oxysporum, Macrophomina phaseolina, and Botrytis sp. was inoculated and incubated at room temperature for 3 to 4 days.
Results and Discussion
The optical properties of AgNP salter when particles aggregate and the conduction of electrons near every particle surface become delocalized and are shared among neighbouring particles. Un-aggregated silver nanoparticles have a yellow colour in solution whereas aggregated particles appear in grey colour [14]. The formation of silver nanoparticles in the solution of 1 mm silver nitrate and aqueous extract of the Hypericum perforatum plant sample was confirmed by a change in the colour to reddish brown, while the controls retained the original colour of the extract (Figure 1). The pale yellow colour appears immediately after the addition of the aqueous plant extract, and the reaction is completed in about 2 hours (Figure 1) [15].
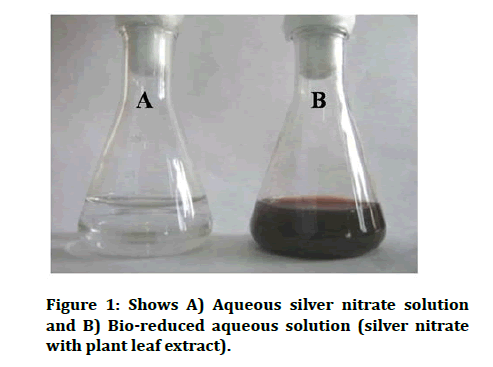
Figure 1: Shows A) Aqueous silver nitrate solution and B) Bio-reduced aqueous solution (silver nitrate with plant leaf extract).
The kinetics of the decrease of aqueous silver nitrate in the reaction was followed by UV-visible spectroscopy (Figure 2) [16,17].
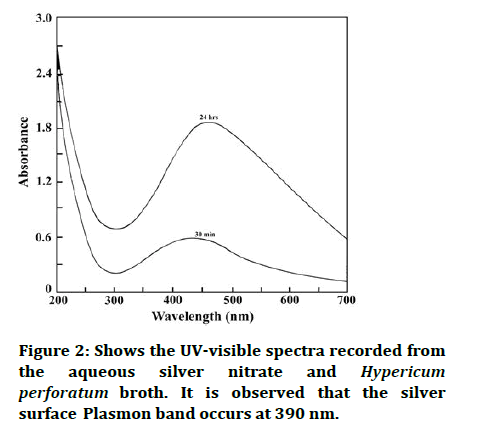
Figure 2: Shows the UV-visible spectra recorded from the aqueous silver nitrate and Hypericum perforatum broth. It is observed that the silver surface Plasmon band occurs at 390 nm.
Developments in Scanning Electron Microscopy (SEM) allow the high resolution imaging of single NPs with sizes below 10 nm. The SEM Examination in transmission Mode (T-SEM) of NPs on thin film bolsters has numerous benefits when compared to the analysis of NPs on bulk substrates [18]. The SEM studies confirmed the formation of silver particles in the size range of 20-100 nm a clear indication of the formation of silver nanoparticles (Figure 3).
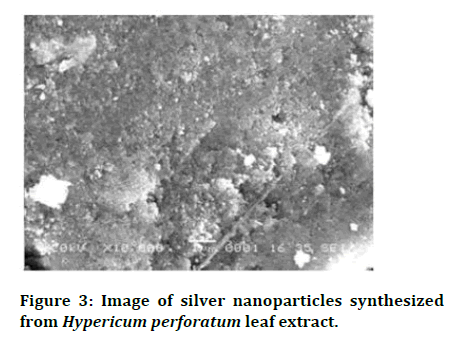
Figure 3: Image of silver nanoparticles synthesized from Hypericum perforatum leaf extract.
X-ray diffraction depends on constructive interference of monochromatic x-rays and a crystalline sample. The nanoparticles synthesized in this method are characterized using powder XRD to confirm the particles as silver and to know the structural information [19]. XRD analysis showed three distinct diffraction peaks and can be indexed 2θ values of 111, 200, 220 crystalline planes of cubic Ag. The average grain size of the silver nanoparticles formed in the bio-reduction process was determined using scherr’s formula, d=(0.9λ*180˚)/β cosθπ and is estimated to be 35 nm (Figure 4) [20].
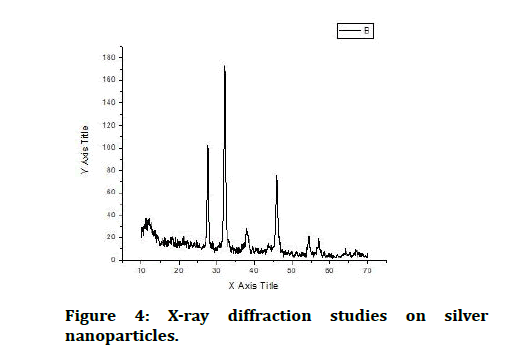
Figure 4: X-ray diffraction studies on silver nanoparticles.
Silver Nanoparticles (AgNPs) possess several applications, such as sensors, catalysts, anticancer agents and antimicrobial agents. AgNPs have exhibited inhibition activity against bacteria, fungi and viruses. In the current study AgNO3 and silver nanoparticles have potentially inhibited the growth of Fusarium oxysporum, Macrophomina phaseolina and Botrytis sp. with the increasing concentration. The zone of inhibition was viewed clearly with the increase in the concentration of AgNO3 and silver nanoparticles in Figure 5 and 6.
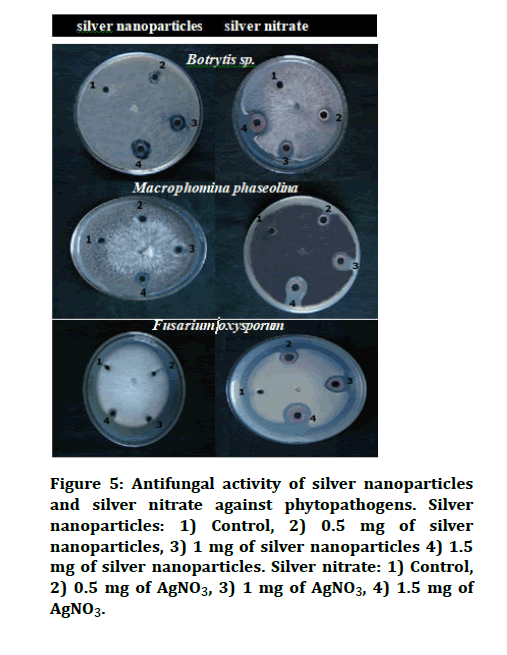
Figure 5: Antifungal activity of silver nanoparticles and silver nitrate against phytopathogens. Silver nanoparticles: 1) Control, 2) 0.5 mg of silver nanoparticles, 3) 1 mg of silver nanoparticles 4) 1.5 mg of silver nanoparticles. Silver nitrate: 1) Control, 2) 0.5 mg of AgNO3, 3) 1 mg of AgNO3, 4) 1.5 mg of AgNO3.
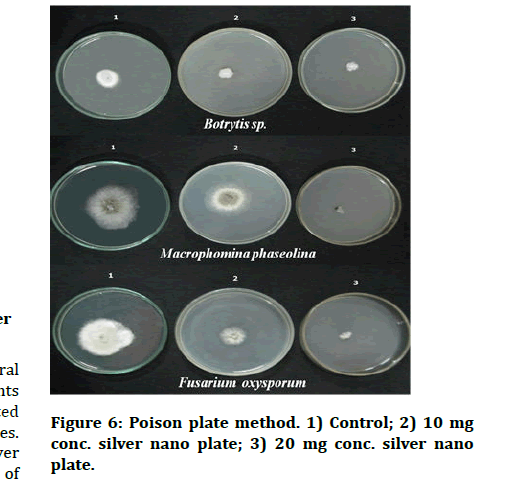
Figure 6: Poison plate method. 1) Control; 2) 10 mg conc. silver nano plate; 3) 20 mg conc. silver nano plate.
In the poison plate method, the radial growth of test pathogens was measured at 10 and 20 mg concentrations. The radial growth of Fusarium oxysporum, Macrophominaphaseolina and Botrytis sp. was recorded as 1.4, 1.0, and 0.9 cm in H. perforatum AgNps (10 mg) plate respectively Figure 6. However, silver nanoparticles showed effective inhibitory action with increasing concentration against Fusarium oxysporum and Macrophominaphaseolin which is concordant with the previous reports of AgNPs from H. perforatum against Candida spp [21,22]. We have found that the silver nanoparticles synthesized in our study effectively inhibited the growth of phytopathogenic fungi like Fusarium oxysporum, Macrophominaphaseolina and Botrytis sp. Silver Nanoparticles (AgNPs) biosynthesized by Hypericum perforatum have been recognized as promising tools to combat parasitic fungi [23].
Conclusion
The silver nanoparticles synthesized from H. perforatum effectively hindered the growth of phytopathogenic fungi. Other biological potentialities, such as antibacterial and anticancer activities of Hypericum perforatum mediated nanoparticles could be also studied in the future.
References
- Modena MM, Ruhle B, Burg TP, et al. Nanoparticle characterization: What to measure? Adv Mater 2019; 31:1901556.
- Rajendran NK, Kumar SS, Houreld NN, et al. A review on nanoparticle based treatment for wound healing. J Drug Delivery Sci Technol 2018; 44:421-430.
- Cunningham FJ, Goh NS, Demirer GS, et al. Nanoparticle mediated delivery towards advancing plant genetic engineering. Trends biotechnol 2018; 36:882-897.
- Feng Y, Marusak KE, You L, et al. Biosynthetic transition metal chalcogenide semiconductor nanoparticles: Progress in synthesis, property control and applications. Curr Opin Colloid Interface Sci 2018; 38:190-203.
- Seddighi NS, Salari S, Izadi AR. Evaluation of antifungal effect of iron oxide nanoparticles against different candida species. Iet Nano Bio Technol 2017; 11:883-888.
- Ahmad S, Munir S, Zeb N, et al. Green nanotechnology: A review on green synthesis of silver nanoparticles an eco-friendly approach. Int J Nanomedicine 2019; 14:5087.
- Ghotekar S, Pagar T, Pansambal S, et al. A Review on green synthesis of sulphur nanoparticles via plant extract, characterization and its Applications. Adv J Chem Section B 2020; 128-143.
- Miri A, Mahdinejad N, Ebrahimy O, et al. Zinc oxide nanoparticles: Biosynthesis, characterization, antifungal and cytotoxic activity. Mater Sci Eng C Mater Biol Appl 2019; 104:109981.
- Serrano Nino JC, Contreras Martinez CA, Solis Pacheco JR, et al. Optimization of the byosynthesis of gold nanoparticles using Hypericum perforatum and evaluation of their antimicrobial activity. Mex J Chem Eng 2020; 19:889-902.
- Roy A, Bulut O, Some S, et al. Green synthesis of silver nanoparticles: Biomolecule nanoparticle organizations targeting antimicrobial activity. RSC adv 2019; 9:2673-2702.
- Bhushan M, Jha R, Bhardwaj R. Reduced band gap and diffusion controlled spherical n-type ZnS nanoparticles for absorption of UV-Visible region of solar spectrum. J Phys Chem Solids 2019; 135:109021.
- Saravanan M, Barik SK, MubarakAli D, et al. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb pathog 2018; 116:221-226.
- Mehta BK, Chhajlani M, Shrivastava BD. Green synthesis of silver nanoparticles and their characterization by XRD. J Phys Conf Ser 2017; 836:012050.
- Oliveira AI, Pinho C, Fonte P, et al. Development, characterization, antioxidant and hepatoprotective properties of poly (Ɛ-caprolactone) nanoparticles loaded with a neuro protective fraction of Hypericum perforatum. Int J Biol Macromol 2018; 110:185-196.
- Ro G, Choi Y, Kang M, et al. Novel colour filters for the correction of red green colour vision deficiency based on the localized surface plasmon resonance effect of Au nanoparticles. Nanotechnol 2019; 30:405706.
- Hassanien AS, Khatoon UT. Synthesis and characterization of stable silver nanoparticles, AgNPs: Discussion on the applications of AgNPs as antimicrobial agents. Physica B Condens Matter 2019; 554:21-30.
- Karami C, Alizadeh A, Taher MA, et al. UV-visible spectroscopy detection of iron (III) ion on modified gold nanoparticles with a hydroxamic acid. J Appl Spectrosc 2016; 83:687-693.
- Amirjani A, Koochak NN, Haghshenas DF. Investigating the shape and size dependent optical properties of silver nanostructures using UV–visible spectroscopy. J Chem Educ 2019; 96:2584-2589.
- Verma P, Maheshwari SK. Preparation of sliver and selenium nanoparticles and its characterization by dynamic light scattering and scanning electron microscopy. J Microsc Ultrastruct 2018; 6:182.
- LaGrow AP, Besenhard MO, Hodzic A, et al. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron x ray diffraction in solution. Nanoscale 2019; 11:6620-6628.
- Mustapha S, Ndamitso MM, Abdulkareem AS, et al. Comparative study of crystallite size using Williamson Hall and Debye Scherrer plots for ZnO nanoparticles. Adv Nat Sci Nanoscience Nanotechnol 2019; 10:045013.
- Muthamil S, Devi VA, Balasubramaniam B, et al. Green synthesized silver nanoparticles demonstrating enhanced in vitro and in vivo anti-biofilm activity against Candida spp. J basic microbiol 2018; 58:343-357.
- Ozgen A, Bilgic E, Aydin SG, et al. Characterization of biosynthesized silver nanoparticles using Hypericum perforatum leaf and determination of their antibacterial activity. Medicine 2019; 8:503-507.
Author Info
Chandran Satheesh Kumar1*, Srinivasagam Raja Sankar2, Kaliyaperumal3 and Prabu K4
1Department of Anatomy,Bharath Institute of Higher Education and Research, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Melmaruvathur, Chennai, Tamil Nadu, India2Department of Anatomy, Melmaruvathur Adhiparasakthi Institute of Medical Sciences and Research, Melmaruvathur, Tamil Nadu, India
3Department of Anatomy, Velammal Medical College, Madurai, Tamil Nadu, India
4Department of Anatomy, Sree Balaji Medical College, Chennai, Tamil Nadu, India
Citation: Chandran Satheesh Kumar, Srinivasagam Raja Sankar, Kaliyaperumal, Prabu K, Green Synthesis of Silver Nanoparticles from Hypericum Perforatum and Evaluation of Antifungal Activity, J Res Med Dent Sci, 2022, 10 (12): 047-051
Received: 03-Oct-2022, Manuscript No. JRMDS-22-77103; , Pre QC No. JRMDS-22-77103(PQ); Editor assigned: 05-Oct-2022, Pre QC No. JRMDS-22-77103(PQ); Reviewed: 17-Oct-2022, QC No. JRMDS-22-77103; Revised: 19-Dec-2022, Manuscript No. JRMDS-22-77103(R); Published: 26-Dec-2022
