Review Article - (2022) Volume 10, Issue 8
Identifying Diagnosis of a Pandemic COVID-19
Sirjan Singh*, Vasant Wagh and Swarupa Chakole
*Correspondence: Sirjan Singh, Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (Deemed to be University), Wardha, Maharashtra, India, Email:
Abstract
In December 2019, the Coronavirus Disease (COVID-19) was found in Hubei Province, China. SARS-CoV-2 is a virus affecting respiratory system that causes coryza like symptoms along with risk of SARS in many. Moreover, investigation of these deadly disease patients has unveiled that the virus can cause symptoms hampering systems other than respiratory. For pandemic prevention and control, prompt and on point spotting of SARS CoV-2 infestation is crucial. Determination can help contain COVID-19 by allowing for quick deployment of control measures such patient recognition, segregation, and contact tracing. The quick and secure recognition of SARS CoV-2, facilitated by active Reverse Transcription–Polymerase Chain Reaction (RT–PCR), is the initial leap to managing COVID-19. Given the benefits and limits of present diagnostics platforms, as well as their solitary result merits, scrutinizing outcomes must be thoroughly reviewed prior to reaching decisions in clinical and non-clinical situations. Conclusion: COVID-19 diagnostic testing is critical for detecting the virus, apprehension of its epidemiology, managing cases, and preventing transmission. Future efforts to build novel diagnostic platforms could pay off if the scrutiny are exact and simple to implement, deliver outcomes quickly, and are inexpensive to mass distribute. Given the benefits and limits of present scrutiny platforms, as well as their solitary result values, testing outcomes must be thoroughly reviewed before putting it out in clinical and non-clinical situations. In the regards to disease transmission and prevention, this Review provides a thorough ladder for diagnostic strategies. It's a basic scientific primer.Keywords
COVID-19, Diagnostic techniques, RT-PCRIntroduction
In the December month of year 2019, the novel Corona-virus Disease (COVID-19) was found in Hubei Province, China [1]. A group of individuals with coryza like symptoms and shortness of breath were admitted. Infection and deaths devastating countries and demolishing communities.
SARS-CoV-2 is a virus affecting respiratory system that causes coryza like symptoms along with risk of SARS [1]. However, investigation of COVID-19 diseased has unveiled that the virus can cause symptoms hampering systems other than respiratory, in addition inflammatory problems in various organs, widening the scope of similar clinical signs [2]. According to current data, COVID-19 infection is seen in all demographics around the globe; however particular endemic individuals are at much raised risk of morbid disease. In regards to the CDC, geriatric age group, consisting as those over the age of 65, are more vulnerable to serious disease than younger people. Patients harbouring major medical illnesses, such as cardiovascular disease, diabetes, or cancer and COPD, are also at a greater risk of grave consequences. There is no indication that youngsters are more prone to infection at this time, although there does appear to be a link between men and a more serious category of the infection and its subsequent prognosis. For pandemic prevention and control, prompt and on point presence of SARS CoV-2 infestation is imminent.
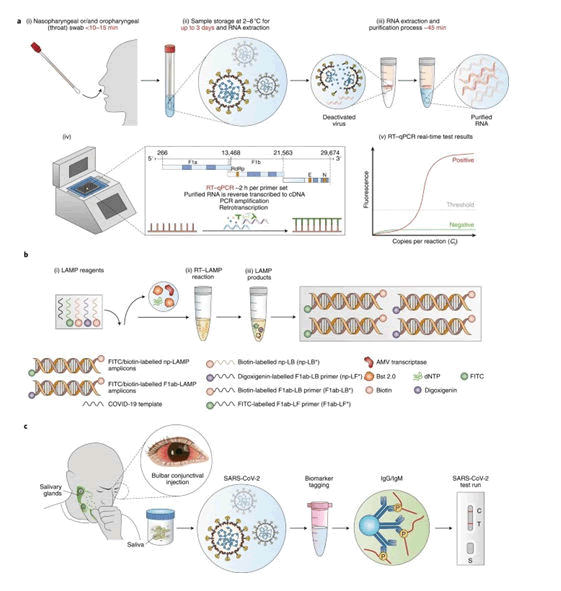
Recuperation and removal of the virus is speculated to be seen when ≥ 2 negative oral swabs are found in a known case or infected person. New studies, however, has rumoured the absolute removal of the virus infectivity in given cases as rectal swabs and culture study of blood may stay positive even after possessing negative oropharyngeal/nasopharyngeal swabs and is of belief that the prominent modes of transmission of the virus include droplets (respiratory), body secretions, feco-oral, human contact, and transmission through surrounding fomites (Figure 1).
Figure 1: RT–PCR and LAMP assays for detection of SARS-CoV-2 infection.
Literature Review
Given the immense transmission capability of the disease and its serious nature, specific monitoring and assessment is utmost important for forming a prompt response. Diagnostics can help contain COVID-19 by allowing for quick deployment of control measures such patient recognition, segregation, and contact tracing.
Another biggest hurdle was posed by the sampling selection and manner of transport. Majority being formed by molecular and serological specimens and they are crucial for disease diagnosis. The initial stage of sample gathering and pre-analytical processing is the most critical time for proper sample management and transfer. Various studies have come to the result depicting the benefit of high quality sample management pose to the positive and better outcome of the sample processing. Defect in transport and storage can lead to invalid results and hamper diagnostic techniques leading to repeat testing and delay in proper management. At present the most popularly used samples are the oropharyngeal and nasopharyngeal swabs.
Clinical presentation of the individual must be used in our favour to the aid the diagnosis. On admission, important laboratory parameters consist of leucocytes that are deranged from the normal range, neutrophils that are raised with regards to the acceptable range, lymphocytes, haemoglobin, and platelets that are decreased. Raised aspartate amino-transferase, alanine amino-transferase, CRP, C. kinase, L-dehydrogenase, BUN, and S. creatinine levels are all possible liver abnormalities. Procalcitonin levels may be over the usual range given the infection index.
In virally imbibed patients, radiological evidence holds a great contribution in the diagnosis of pneumonia. Over 75 percent and 71 percent of adult patients, respectively, had bilateral and multi-lobe lung damage. For the determination of COVID-19 linked pneumonia in paediatric age group, the following criteria for elevated respiratory rate should be used: 60 times/min for less than 2 m. old; 50 times/min for 2-12 m. old; 40 times/min for 1-5 years old; 30 times/min for >5 year old.
Other viral illnesses involving respiratory system caused by the SARS virus, parainfluenza virus, adeno-virus, RS virus, influenza-virus and meta-pneumovirus can be utilised in the differential diagnosis. Another important type of misleading presentation is mycoplasma pneumonia. For the utmost tracing of COVID-19, exposure due to epidemiological presence and culture done for blood and sputum samples can play a major role.
The identification of the infectious agent, as with any other infection, is the gold standard for diagnosis. In the case of viral infections, this identification can be accomplished by using electron microscopy to visualise viral particles or light microscopy to identify intracellular viral inclusions. In order to examine in vitro virus replication, tissue cultures are required. These procedures necessitate technology that is often only found in research facilities. Immune enzymatic assays or agglutination tests for determining viral antigens and nucleic acid amplification tests for detecting virus genomic material are accessible in commercial laboratories [3-7].
The identification of a specific immune system response is an indirect technique to diagnose viral infections. The humoral response, or antibody production, is the most straightforward method for diagnosing infectious diseases. Antibodies directed against distinct sections of viruses can be identified using a variety of ways.
For the sake of discussion and all practicality, three major types of tests for COVID-19 diagnostic purposes used are: molecular RT-Polymerase Chain Reaction, serological investigations and rapid antigen or antibody tests. The struggle at hand is that each of these tests can be applied at specific stage of infectivity only. Hence, the effort for multi discipline approach and raised cost effectiveness and man power along with training effort for the particular technique.
One of the main aim for all this struggle and race to come up with so many vast variety of diagnostic techniques is to develop a screening strategy to be implemented for efficient screening of the pandemic causing virus. Techniques can be utilized to be made as surveillance for all the infected and non-infected personnel.
Current diagnostic techniques
The COVID-19 pandemic proposes a provocation for the favourable and secure surgical relief of patients. Al-Jabir et al. summarize the huge outcome of the global phenomenon, bridging across all surgical specialties around the globe, highlighting the need for urgency. Because molecular approaches may target and detect specific diseases, they are more suitable for accurate diagnoses than syndromic investigations and CT-scans. Researchers need genome sequencing to create primers and probe sequences for PCR and other nucleic acid testing.
The quick and reliable spotting of SARS CoV-2, facilitated by RT-PCR, is the initial leap towards managing COVID-19. SARS CoV-2 nucleic acids are detected in nasopharyngeal fluids by RT-Polymerase Chain Reaction.8 Asymptomatic sick people, whose viral discharge can unwittingly open out the illness to the aged and others with disease comorbidities, are tested to halt infectious growth between people and communities [9]. The first step in limiting the COVID-19 epidemic is accurate virus detection [10]. Defects jeopardise public safety by allowing illness to spread thanks to false-negative test results [11-14]. The need to improve test sensitivity and specificity is still pressing. Serological testing adds to virus detection by indicating a previous infestation that could be used for healing purposes. Antibodies are detected utilising a qualitative detection of IgG or IgM ABs in an Enzyme-Linked Immunosorbent Assay (ELISA) [15].
Due sudden surge in patients and requirement for diagnosis of suspected individuals, there came a demand for a rapid and economic disease determination strategy to be part of and facilitate in the ever growing diagnostic program. Amidst all the chaos came the Rapid antigen and rapid antibody testing kit. It has rapid outcome time of 15-30 mins. Saving time and money. Easy to preach and perform. No special training or personnel required. Based on the concept of Lateral Flow Immunoassay (LFIA) so as to directly harbour the presence of viral proteins and human antibodies against SARS CoV-2 antigen. The themes behind the Lateral Flow Immunoassay (LFIA) rapid antigen test is quite simple and consist of bond between antigen and antibody being present on the oropharyngeal and nasopharyngeal wall, which is transported by swab buffer through capillary flow. The exults are readily available and can be interpreted by naked eye in gist of time and can be implemented without any special equipment or process of specimens. Its downfall comes in its low specificity and sensitivity.
ABs, Nucleic acid and protein dependent determination are current methods in present determination procedures for the SARS CoV-2 pandemic, even though viral genomic observation by RT-Polymerase Chain Reaction (RT–PCR) remains of utmost importance [7]. In contrast to present accessible immunological testing, nucleic acid assays have enhanced reactivity and accuracy for virus detection.
Discrepancy in viral RNA genomic coding can change real-time RT-Polymerase Chain Reaction (RT-PCR) outcome using primers targeting various viral genomic regions. Even more, due to viral mutation, false-negative results may occur [8]. Sample repository, poor-natured nucleic acid clarification, expense, and wait durations are all disadvantages of RT-PCR tests [6]. Despite these drawbacks, the RT-PCR test remains the best and most effective for diagnosing Severe Acute Respiratory Syndrome (SARS) CoV-2 [16-18].
Anti-body against Severe Acute Respiratory Syndrome (SARS) CoV-2 are produced as a main immunological reaction to infection. By day 7, up to 50% of infected individuals have neutralising antibodies, and by day 14, all infected individuals have neutralising antibodies. For SARS-CoV-2 diagnoses, serological researches are a replacement to RT-PCR. When real-time PCR and serological tests are used together, the rate of positive virus identification rises dramatically. In most people, IgM antibodies levels rise during the initial week following Severe Acute Respiratory Syndrome (SARS) CoV-2 infection, peak after two weeks, and subsequently decline to near-background levels. After one week, IgG antibody is traceable and stays at elevated level for a long time. IgG, on the other hand, is detectable after one week, remains raised for a long time, perhaps up to 48 days, and may work out to protect against resurgence. Between 4 and 10 days post infection, IgA responses appear. The presence of serum IgA 57, as well as IgG and IgM 58, is a diagnostic predictor.
Despite the RT-PCR's excellent sensitivity, patients with a minimal viral load are frequently misdiagnosed. Furthermore, this technique necessitates confirmatory investigations, skilled staff, and overpriced tools and reagents, all of which bounds its use, especially in low-income nations. Other approaches have been made to circumvent the constraints of RT-Polymerase Chain Reaction for these objectives. CRISPR/Cas-based assays and iso-thermal enhancement methods are two of the promising economic diagnostic technologies that can be utilized to diagnose COVID-19 infection in economically poor performing, substantially minimal income countries. Furthermore, more accurate molecular approaches, including as ddPCR and biosensors, are starting to be recognized by overseas authorities and employed for COVID-19 infection diagnosis as well as viral load monitoring in admitted patients or confined individuals.
In addition, a quick distinctive assay was formed to determine the existence of viral antigens produced by SARS CoV-2 in specimens from infected persons' respiratory tracts. Antigen in the sample secure to antibodies mounted to a paper strip contained in a plastic container for this experiment. Within half an hour, this reaction produces a clearly discernible signal. Because the antigen(s) discovered are only expressed when the virus is presently duplicating, the procedures can be used to diagnose acute or new infection [19].
Another advance technique in this regards is the viral culture that had come as a phenomenal technique in identification of SARS CoV-2. Though one has to cope with long period of strict viral replication to obtain the result in vitro, it has come up as a milestone in the diagnostic aid. It was initially successfully done by Zhu they then assessed the efficacy of virus and its potential cytopathic effect and electron microscopy.
On note of electron microscopy, it is an upcoming advancement for detection of structural features of the virus and its identification. Overall these techniques can become a major thing for the observation of viral features and its surest detection platforms.
Discussion
Limitations
Despite transparent and worldwide alliance between national and international organizations, variability in diagnostic criteria leads to a misleading epidemiology scenario. COVID-19 is now diagnosed using a combination of chest CT scans and RT–PCR data. RT–PCR testing accounts for the great number of scrutiny done in the task place or in academics outside of a clinical environment. Because RT–PCR testing is so common, it's crucial to look into what this test can tell doctors and policymakers. Future observation methods can enhance on the current model by finding the flaws in this testing platform. Along with all investigatory techniques it is of prime significance to take into fact the clinical history, modality of infection, type of sample to be procured in order to come up with an appropriate diagnosis of COVID-19 infection and then carry on with further appropriate approach to get proper management keeping in mind the time constraint, co-morbidities and effectiveness of it all.
Future
Future efforts to build novel diagnostic platforms could pay off if the scrutiny are exact and simple to implement, deliver outcomes quickly, and are inexpensive to mass distribute. Given the benefits and limits of present scrutiny platforms, as well as their solitary result values, testing outcomes must be thoroughly reviewed before putting it out in clinical and non-clinical situations. To anticipate future epidemics, new technologies like as ergonomic, economic mass pooling must be considered.
Conclusion
Infection control has previously been impeded by a lack of testing accessibility; nevertheless, testing of this unique virus is rapidly rising. COVID-19 diagnostic testing is critical for detecting the virus, realizing its epidemiology, managing cases, and preventing spread.
As likely, academic scientists and biotechnologists have been tasked with describing additional SARS CoV-2 strains in order to enhance ABs and anti-gen based tests' cluster-based specificity and sensitivity. Majorly, nanomaterial-based virus detection technology can aid in the upcoming of COVID-19 detection assays that are high-sensitivity, easy and economical in the event of a pandemic. All in all, in the given time period of disease upsurge, the scientific and medical community has devised such brilliant techniques from RT-PCR to electron microscopy to aid the process of diagnostic of the COVID-19 infection. No doubt that the community at present needs a quick, economic and efficient way to detect the presence of virus, but only by integrating all the techniques and modalities only we can maintain a strict and accurate diagnosis and management of the deadly virus and effectively manage this global tragic phenomenon.
In the regards to disease spread and elimination, this Review provides an accurate ladder for diagnostic strategies. It's a basic scientific primer for better understanding COVID-19 diagnostic complexity and developing better disease-fighting techniques.
References
- Kevadiya BD, Machhi J, Herskovitz J, et al. Diagnostics for SARS-CoV-2 infections. Nature materials 2021; 20:593-605.
- Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. Jama 2020; 324:782-793.
[Crossref] [Google Scholar] [Pubmed]
- Saegerman C, Gilbert A, Donneau AF, et al. Clinical decision support tool for diagnosis of COVID-19 in hospitals. PloS one 2021; 16:e0247773.
[Crossref] [Google Scholar] [Pubmed]
- Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of coronavirus disease 2019. Clin Infect Dis 2020.
[Crossref] [Google Scholar] [Pubmed]
- Fenollar F, Bouam A, Ballouche M, et al. Evaluation of the Panbio Covid-19 rapid antigen detection test device for the screening of patients with Covid-19. J Clin Microbiol 2020; 59:02589-02620.
[Crossref] [Google Scholar] [Pubmed]
- Raffle AE, Pollock AM, Harding-Edgar L. COVID-19 mass testing programmes. bmj 2020; 370.
[Crossref] [Google Scholar] [Pubmed]
- Rosenberg ES, Hall EW, Rosenthal EM, et al. Monitoring coronavirus disease 2019 (COVID-19) through trends in influenza-like illness, laboratory-confirmed influenza, and COVID-19–New York state, excluding New York City. Clin Infect Dis 2021;72:144-147.
[Crossref] [Google Scholar] [Pubmed]
- Zhai P, Ding Y, Wu X, et al. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents 2020;55:105955.
- Tang YW, Schmitz JE, Persing DH, et al. Laboratory diagnosis of COVID-19: current issues and challenges. J Clin Microbiol 2020; 58:00512-00520.
[Crossref] [Google Scholar] [Pubmed]
- Al-Jabir A, Kerwan A, Nicola M, et al. Impact of the Coronavirus (COVID-19) pandemic on surgical practice-Part 2 (surgical prioritisation). Int J Surg 2020; 79:233-248.
[Crossref] [Google Scholar] [Pubmed]
- Babidge WJ, Tivey DR, Kovoor JG, et al. Surgery triage during the COVIDâ?19 pandemic. ANZ J Surg 2020; 90:1558-1565.
[Crossref] [Google Scholar] [Pubmed]
- Nicola M, Sohrabi C, Mathew G, et al. Health policy and leadership models during the COVID-19 pandemic: A review. Int J Surg 2020; 81:122-129.
[Crossref] [Google Scholar] [Pubmed]
- Nicola M, O'Neill N, Sohrabi C, et al. Evidence based management guideline for the COVID-19 pandemic-Review article. In J Surg 2020; 77:206-216.
[Crossref] [Google Scholar] [Pubmed]
- Yi H. 2019 novel coronavirus is undergoing active recombination. Clin Infectious Dis 2020; 71:884-887.
[Crossref] [Google Scholar] [Pubmed]
- Young BE, Ong SW, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. Jama 2020; 323:1488-94.
[Crossref] [Google Scholar] [Pubmed]
- Liu Y, Chen H, Tang K, et al. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J infect 2020; 10.
[Google Scholar] [Pubmed]
- Zhu Y, Gao ZH, Liu YL, et al. Clinical and CT imaging features of 2019 novel coronavirus disease (COVID-19). J infect 2020; 81:147-178.
[Crossref] [Google Scholar] [Pubmed]
- Wang FS, Zhang C. What to do next to control the 2019-nCoV epidemic? The Lancet 2020; 395:391-393.
[Crossref] [Google Scholar] [Pubmed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. The lancet 2020; 395:507-513.
[Crossref] [Google Scholar] [Pubmed]
Author Info
Sirjan Singh*, Vasant Wagh and Swarupa Chakole
Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences (Deemed to be University), Wardha, Maharashtra, IndiaCitation: Sirjan Singh,Vasant Wagh, Swarupa Chakole, Identifying Diagnosis of a Pandemic COVID-19, J Res Med Dent Sci, 2022, 10 (7): 000-000.
Received: 27-May-2022, Manuscript No. JRMDS-22-49585; , Pre QC No. JRMDS-22-49585; Editor assigned: 31-May-2022, Pre QC No. JRMDS-22-49585; Reviewed: 14-Jun-2022, QC No. JRMDS-22-49585; Revised: 28-Jul-2022, Manuscript No. JRMDS-22-49585; Published: 06-Aug-2022