Research Article - (2022) Volume 10, Issue 12
Immunohistochemical Based Study on Frequency of HPV in Oral Squamous Cell Carcinoma Biopsies of Iraqi Kurdistan Patients
Aween Auda Ablahad1*, Hashim Dawood Mousa2 and Jalal Ali Jalal3
*Correspondence: Dr. Aween Auda Ablahad, Department of Oral and Maxillofacial Surgery, College of Dentistry, University of Duhok, Duhok, Iraq, Email:
Abstract
Introduction: Implication of Human Papilloma Virus (HPV) in the carcinogenesis of Oral Squamous Cell Carcinoma (OSCC) is debatable subject, p16 overexpression indicates active HPV infection in Oropharyngeal Squamous Cell Carcinoma (OPSCC) but in OSCC such relation still needs to be studied. Therefore, we aimed to evaluate the frequency of HPV in OSCC patients in the capital of Kurdistan region of Iraq and its concordance with p16 overexpression.
Materials and methods: We retrieved eighty-six Formalin Fixed Paraffin Embedded (FFPE) samples of OSCC from multi large pathological centres that located in the capital of Kurdistan, we utilized Immunohistochemistry (IHC) to detect the HPV by anti-HPV high risk antibody correlated it with p16 overexpression, besides, twenty FFPE samples of healthy gingiva were used as control in this study.
Statistical analysis: Chi square and fisher’s exact tests were used for correlating the HPV status and p16 overexpression with clinicopathogical patient’s data. The concordance between HPV and p16 overexpression was evaluated by kappa agreement and spearman rank correlation.
Results: The frequency of HPV in OSCC patients were 15.1%, tongue was the most common site affected by HPV infection, other patient data including age, gender, grade and stage did not show significant correlation with neither anti-HPV nor p16 antibodies. The concordance level between p16 overexpression and the HPV status according to kappa agreement was (κ=0.221, p=0.034), Moreover, the correlation according to spearman correlation coefficient was (r=0.229, p=0.034), with 46.15% sensitivity and 80.82% specificity.
Conclusion: We concluded that HPV infection is still low in Erbil and p16 biomarker has only diminutive significance as a predictor of HPV infection in the OSCC patients.
Keywords
Human papilloma virus, p16, Oral squamous cell carcinoma
Introduction
From the epidemiologic perspective, a curious observation over the last elapsed few decades was the synchronously rising in the incidence of HPV positive OSCC [1]. The causality for such escalation had being related to changes in the sexual attitude in the population across various countries [2].
Worldwide, the exact distribution and prevalence of HPV positive OSCC needs to be estimated as the HPV incidence varies through different nations and the geographical locations, markedly, HPV driven OSCC cases are sharply expanding in the Europe, USA and South Asian countries, while in developing countries, such proportions are hard to be appraised as health institutions lack routine testing for HPV infection [3].
The carcinogenesis of HPV driven OPSCC involves binding of the viral protein E7 to retinoblastoma protein, in activating and degranulation it, which results in prompt increases the p16 expression [4]. Interestingly, HPV positive and p16 overexpression have been proved to be correlated with better prognosis in patients with of OPSCC [5].
In OPSCC, p16 is regarded as a surrogate marker for HPV infection, however, In OSCC, the subject is controversial, and p16 has been demonstrated as a poor indicator for HPV infection because of its poor predictive value and low sensitivity [6]. Moreover, evidences indicated that overexpression of p16 could yield false positive prediction for HPV status in 5% to 20% of OSCC patients [7].
Unfortunately, up to date, only scant data are documented about HPV status in OSCC in Kurdistan that prompted us to appraise, identify and synthesize the best evidences in an attempt to answer the two foremost questions. First: What is the frequency of high risk HPV in OSCC in Iraqi Kurdistan inhabitants? Second: Could p16 biomarker used as a standalone test for prediction of HPV positivity in our OSCC patients?
Materials and Methods
Samples selection: In this case control retrospective study, we retrieved 86 FFPE samples of OSCC form rizgary teaching hospital and private hospitals in Kurdistan region of Iraq, in addition to 20 FFPE samples of healthy gingiva as control, in the period between 1/1/2016 and 30/8/2021, any OSCC sample that had enough tissue with clinic pathological information was included in the study. Samples with previous radiotherapy or chemotherapy were excluded from this work. The current work was approved and performed at college of dentistry/university of duhok; the practical part was performed between Februarys to June 2022.
Methods of H and E and IHC stains: From each paraffin block of healthy gingiva and OSCC samples, three sections with 4 μm thickness were obtained, each mounted on a new glass slide, first section was stained with H and E stain to make re-evaluation of the diagnosis and tumour grade that were previously written in the reports. The other two sections were immunohistochemically stained with p16 monoclonal antibody (Dakocytomatation, MIB-1Clone, 1:300 and anti-HPV high risk monoclonal antibody which reacts with HPV subtypes 6; 11; 16; 18; 31; 33; 42; 51; 52; 56; and 58 (dakocytomatation, K1H8 Clone, 1:50 respectively, using auto strainer and envision TM FLEX detection kit from dako (dako/ denmark.
Criteria for IHC evaluation: P16 expression was determined as positive when there was diffuse and strong nuclear and cytoplasmic staining in >70% of the cancer cells [8]. For HPV evaluation, the sample was considered positive when there was immune reactivity in the nuclear and/or cytoplasm of the tumour cells [9]. All the H and E and IHC stained slides were viewed by two pathologists and photographed using a light microscopy from Lieca/Germany in central public lab (Figure 1).
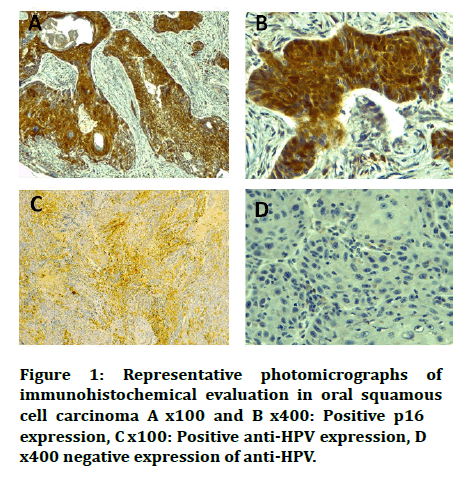
Figure 1: Representative photomicrographs of immunohistochemical evaluation in oral squamous cell carcinoma A x100 and B x400: Positive p16 expression, C x100: Positive anti-HPV expression, D x400 negative expression of anti-HPV.
Statistical analyses: Our data were analysed using IBM SPSS Statistics (version: 28.0.1.1.14. Associations of p16 expression and anti HPV expression with clinic pathological variables were tested using Chi-square and Fisher’s exact tests, agreement between p16 and Anti- HPV was evaluated on the one hand by Cohen's kappa (denoted by κ), secondly by Spearman’s rank order correlation (denoted by r),v alues of κ<0.20 were judged as “poor “, values between 0.21 and 0.40 as “fair”, we applied the same rating for the correlation coefficients. P value of <0.05 was regarded as statistically significant.
Results
Association of p16 and HPV expression with patient’s data: Regarding OSCC, A total of 86 biopsies was evaluated in this study. The distribution and associations between p16 and HPV and clinic pathological feature of OSCC samples are listed in Table 1. The HPV status showed significant correlation with the site of the tumour, while p16 did not show any significant correlation with the investigated clinic pathological parameters. Importantly, all samples of healthy gingiva were negative for both p16 and HPV expression.
| Variables | OSCC | P16 | HPV | ||
|---|---|---|---|---|---|
| n | P16-n | P16+n | HPV-n | HPV+n | |
| Total | 86 | 66 | 20 | 73 | 13 |
| Age | |||||
| <60 | 37 | 26 | 11 | 32 | 5 |
| ≥ 60 | 49 | 40 | 9 | 41 | 8 |
| P | 0.303 | 0.771 | |||
| Gender | |||||
| Male | 58 | 47 | 11 | 51 | 7 |
| Female | 28 | 19 | 9 | 22 | 6 |
| P | 0.276 | 0.337 | |||
| Site | |||||
| Lip | 44 | 37 | 7 | 42 | 2 |
| Tongue | 20 | 14 | 6 | 14 | 6 |
| Palate | 16 | 10 | 6 | 11 | 5 |
| Others | 6 | 5 | 1 | 6 | 0 |
| P | 0.254* | 0.006* | |||
| Grade | |||||
| G1 | 26 | 19 | 7 | 22 | 4 |
| G2 | 56 | 44 | 12 | 47 | 9 |
| G3 | 4 | 3 | 1 | 4 | 0 |
| P | 0.821* | 1.00* | |||
| PT Stage | |||||
| T1+T2 | 58 | 47 | 11 | 49 | 9 |
| T3+T4 | 28 | 19 | 9 | 24 | 4 |
| P | 0.276 | 1 | |||
| G=grade, HPV=human papilloma virus, PT stage=Pathological T stage, n=number, OSCC=oral squamous cell carcinoma, *=Fisher exact test, +=positive, -=negative. | |||||
Correlation of p16 overexpression with HPV status: The distribution of cases is shown in Table 2. The true positive cases were 6 (7%) and true negative cases were 59 (68.6), while false positive cases were 14 (16.3) and false negative cases were 7 (8.1) from total samples of OSCC. According to kappa and spearman’s correlation coefficient, p16 expression and HPV status revealed fair significant level of agreement (κ=0.221, p=0.034) and fair significant correlation (r=0.229, p=0.034. The sensitivity of p16 was 46.15% while specificity was 80.82% (Table 2).
| Cases | HPV | Total, n (%) | |
|---|---|---|---|
| HPV-, | HPV+, | ||
| n (%) | n (%) | ||
| P16 | |||
| P16- | 59 (68.6) | 7 (8.1) | 66 (76.7) |
| P16+ | 14 (16.3) | 6 (7) | 20 (23.3) |
| Total | 73 (84.9) | 13 (15.1) | 86 (100) |
Table 2: Association between HPV status and p16 expression in OSCC cases.
Discussion
Historically, the crucial risk factors associated with OPSCCs were alcohol consumption and usage of tobacco, alarmingly, current epidemiological evidences point out a significant increase in HPV related OPSCC, hence, in 2020 was postulated that HPV driven OPSCCs were exceeded cervical carcinomas in United States of America [10].
In unfortunate, until we elaborated this research, we realized a lack of published data addressing the HPV in OSCC in Kurdistan region of Iraq, our study took into consideration this delimitation, as a consequence, we demonstrated in this multicentre pivotal study, that the causality shift into HPV positive OSCC that observed in the western world has declared itself partly within patients who are diagnosed in Kurdistan hospitals, we evidenced it for the first time by the 15.1% HPV positive OSCC in our study samples. This ratio was not unanticipated, since the socioeconomic status throughout Kurdistan region of Iraq is growing, which could push the region through an epidemiologic transformation. Interestingly, it is hard to compare the prevalence precisely with previous studies because of the remarkable variations in sampling, detection methods, sample size, and including of different anatomical sites across studies [11].
There are variable approaches and methods for identifying the HPV status, clinically; IHC is more routinely utilized because it shows many benefits as it is simple, practical and inexpensive [4,12]. In the current work, we employed both anti HPV high risk antibody and p16 antibody for determination of the HPV status.
Concerning the patient characteristics, only the site of the tumour showed significant results in relation to the anti- HPV expression, tongue was more frequently HPV cancerous developer (7%). This intriguing allocation pattern was corroborated also by panzarella and colleagues [13]. Our and vanshika and colleagues findings revealed that no significant correlations existed between the HPV and age, gender, grade and stage of the tumour [14]. Meanwhile, other studies probed significant correlations with gender and grade of the tumour [15].
The positivity for p16 overexpression (23.3%) was found to be within the range certified by the other authors [6,15,16]. Concerning our set of the p16 positive data, our findings denoted no significant correlations were disclosed between p16 overexpression and all investigated parameters, we thereby confirmed previous results by other authors [8,17-19]. Conversely, vanshika and colleagues demonstrated significant correlation with grade [14]. While, Trinh, et al. found p16 overexpression is significantly correlated with PT stage of the tumour [20].
In OPSCC, p16 overexpressed is proven to be a surrogate indirect biomarker for active HPV infection [21,22]. While in OSCC the subject is debatable. Fair correlation and concordance were found between p16 overexpression and HPV status (r=0.229, p=0.034) (κ=0.221, p=0.034) respectively in our samples which seemed to be in line with Komolmalai, et al. Moreover, we remarkably discovered that 14 (16.3%) patients with p16 overexpression were HPV negative, which agreed with summarizing work of Wang, et al. [23] furthermore, 7 (8.1%) of our patients were p16 negative but HPV positivity was observed.
In the literature, many studies have tried to elucidate this critical correlation; each came with distinct results; in Galvis, et al. Study, all HPV positive cases were found to be negative for p16 overexpression, hence p16 was suggested as non-indicative of HPV infection [24]. Moreover, many other published studies had not indicated correlation between p16 overexpression and HPV status [25-27]. According to Lechner, et al. opinion, p16 overexpression in the lacking of HPV infection in the oral cancer could be attributable to alternate molecular pathways including inactivation of retinoblastoma protein by deletion or mutations and p16 amplification, inversely, OSCC patients with HPV positive/p16 negative could carry deletions or mutations in p16 gene, thus prohibits the p16 from expression [28].
None the less, we are aware of one study by de Lima, et al. which stated that p16 overexpression (with 50% cut off point) acted as an accurate indirect marker of HPV status and it yielded high sensitivity and specificity, they came with conclusion that p16 could be used as a marker of HPV infection [15]. Arsa, et al. reported that the employment of p16 status as a marker for HPV infection could be more accurate in western countries where high frequency of p16 overexpression and HPV positive OSCC found in their population [29].
Conclusion
We came with the following conclusions: First, the frequency of HPV in OSCC patients is still low in Iraqi Kurdistan patients as compared to western countries and smoking yet is the crucial risk factor for developing such tumour. Second, in spite of positive correlation between p16 overexpression and HPV status, the sensitivity of p16 antibody as a determinant for HPV infection was poor. Here in, we reported that p16 is a poor diagnostic tool and recommend not carrying out this test as a predictor of HPV related OSCC for our patients.
Limitations
However, this work has several limitations, we could not include all centres of pathology in Kurdistan, besides, further data gathering is needed as heath system in Kurdistan receives patients also form the other cities of Iraq which may hamper the results.
Acknowledgments
AAA (Author 1) and JAJ (Author 3) were involved in the conceptualization, methodology, investigations, data analysis and writing. HDM (Author 2) involved in analysing of control samples, writing of original draft, review and editing of the manuscript. JAJ and HDM helped in the supervision. All authors read and approved the final manuscript.
References
- Irani S. New insights into oral cancer risk factors and prevention: A review of literature. Int J Prev Med 2020; 11.
- Foy JP, Bertolus C, Boutolleau D, et al. Arguments to support a viral origin of oral squamous cell carcinoma in non-smoker and non-drinker patients. Front Oncol 2020; 10:822.
- Holmes HK, Afrogeh A, Adeola H, et al. Prevalence and distribution of HPV infection and subtypes in oral squamous cell carcinoma in Africa: A systematic review protocol. BMJ open 2021; 11:049922.
- Jiromaru R, Yamamoto H, Yasumatsu R, et al. P16 over expression and RB loss correlate with high risk HPV infection in oropharyngeal squamous cell carcinoma. Histol 2021; 79:358-369.
- Naz F, Verma H, Tanveer N, et al. Demographic profile of p16 Immuno positive and HPV DNA PCR positive oral squamous cell carcinoma in a large cohort of Indian patients. APJCP 2022; 23:529-536.
- Schneider K, Jakobsen KK, Jensen JS, et al. Impact of p16 overexpression on overall and progression free survival outcomes in oral cavity squamous cell carcinomas: A semi national, population based study. Oral Oncol 2020; 111:105031.
- Benzerdjeb N, Tantot J, Blanchet C, et al. Oropharyngeal squamous cell carcinoma: p16/p53 immunohistochemistry as a strong predictor of HPV tumour status. Histopathol 2021; 79:381-390.
- Tokuzen N, Nakashiro KI, Tojo S, et al. Human papillomavirus 16 infection and p16 expression in oral squamous cell carcinoma. Oncol lett 2021; 22:1-6.
- Qiu Q, Li Y, Fan Z, et al. Gene expression analysis of human papillomavirus associated colorectal carcinoma. Bio Med Res Int 2020.
- Ramesh PS, Devegowda D, Singh A, et al. NRF2, p53, and p16: Predictive biomarkers to stratify human papillomavirus associated head and neck cancer patients for de-escalation of cancer therapy. Crit Rev Oncol Hemat 2020; 148:102885.
- Sri S, Ramani P, Premkumar P, et al. Prevalence of Human Papilloma Virus (HPV) 16 and 18 in oral malignant and potentially malignant disorders: A polymerase chain reaction analysis a comparative study. Ann Maxillofac Surg 2021; 11:6.
- De C, Ferreira C, Dufloth R, et al. Correlation of p16 immunohistochemistry with clinical and epidemiological features in oropharyngeal squamous cell carcinoma. PloS One 2021; 16:0253418.
- Panzarella V, Campisi G, Giardina Y, et al. Low frequency of human papillomavirus in strictly site coded oral squamous cell carcinomas, using the latest NHI/SEER ICD systems: A pilot observational study and critical review. Cancers 2021; 13:4595.
- Vanshika S, Preeti A, Sumaira Q, et al. Incidence OF HPV and EBV in oral cancer and their clinic pathological correlation a pilot study of 108 cases. J Oral Biol Craniofac Res 2021; 11:180-184.
- de Lima MA, Cavalcante RB, Da Silva CG, et al. Evaluation of HPV and EBV in OSCC and the expression of p53, p16, E‐cadherin, COX‐2, MYC, and MLH1. Oral Dis 2022; 28:1104-1122.
- Bouland C, Dequanter D, Lechien JR, et al. Prognostic significance of a scoring system combining p16, smoking, and drinking status in a Series of 131 patients with oropharyngeal cancers. Int J Otolaryngol 2021; 2021.
- Agarwal VK, Sharma R, Gahlot GP, et al. Clinical and histopathological correlation of p16 and p53 expression in oral cancer. Indian J Surg Oncol 2021; 12:164-168.
- Komolmalai N, Pongsiriwet S, Lertprasertsuke N, et al. Human papillomavirus 16 and 18 infection in oral cancer in Thailand: A multicentre study. APJCP 2020; 21:3349.
- Blahak J, Zelinka J, Gumulec J, et al. HPV, protein p16 and squamous cell carcinoma of the oral cavity. Biomed Pap Med Fac 2020; 164.
- Trinh JM, Thomas J, Salleron J, et al. Differences in clinical and imaging characteristics between p16 positive non-smokers and p16 positive smokers or p16 negative patients in oropharyngeal carcinoma. Sci Rep 2021; 11:1-1.
- Carlander AF, Jakobsen KK, Bendtsen SK, et al. A contemporary systematic review on repartition of HPV-positivity in oropharyngeal cancer worldwide. Viruses 2021; 13:1326.
- Hammarstedt L, Holzhauser S, Zupancic M, et al. The value of p16 and HPV DNA in non-tonsillar, non-base of tongue oropharyngeal cancer. Acta Oto Laryngol 2022; 141:89-94.
- Wang H, Zhang Y, Bai W, et al. Feasibility of immunohistochemical p16 staining in the diagnosis of human papillomavirus infection in patients with squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Front Oncol 2020; 10:524928.
- Galvis MM, Jardim JF, Kaminagakura E, et al. Expression of cell cycle proteins according to HPV status in oral squamous cell carcinoma affecting young patients: A pilot study. Oral Surg Oral Med Oral Pathol Oral Radiol 2018; 125:317-325.
- Palve V, Bagwan J, Krishnan NM, et al. Detection of high risk human papillomavirus in oral cavity squamous cell carcinoma using multiple analyses and their role in patient survival. J Glob Oncol 2018; 4:1-33.
- Sundberg J, Korytowska M, Burgos PM, et al. Combined testing of p16 tumour suppressor protein and human papillomavirus in patients with oral leukoplakia and oral squamous cell carcinoma. Anticancer Res 2019; 39:1293-1300.
- Yang LQ, Xiao X, Li CX, et al. Human papilloma virus genotypes and p16 expression in oral leukoplakia and squamous cell carcinoma. Int J Clin Exp Pathol 2019; 12:1022.
- Lechner M, Chakravarthy AR, Walter V, et al. Frequent HPV independent p16/INK4A overexpression in head and neck cancer. Oral Oncol 2018; 83:32-37.
- Arsa L, Siripoon T, Trachu N, et al. Discrepancy in p16 expression in patients with HPV associated head and neck squamous cell carcinoma in Thailand: Clinical characteristics and survival outcomes. BMC cancer 2021; 21:1-2.
Author Info
Aween Auda Ablahad1*, Hashim Dawood Mousa2 and Jalal Ali Jalal3
1Department of Oral and Maxillofacial Surgery, College of Dentistry, University of Duhok, Duhok, Iraq2Department of Periodontology and Oral Medicine, College of Dentistry, University of Duhok, Duhok, Iraq
3Department of Basic Sciences and Pathology, College of Medicine, Hawler Medical University, Erbil, Iraq
Citation: Aween Auda Ablahad, Hashim Dawood Mousa, Jalal Ali Jalal, Immunohistochemical Based Study on Frequency of HPV in Oral Squamous Cell Carcinoma Biopsies of Iraqi Kurdistan Patients, 2022, 10 (12): 094-099.
Received: 03-Oct-2022, Manuscript No. JRMDS-22-76279; , Pre QC No. JRMDS-22-76279(PQ); Editor assigned: 07-Oct-2022, Pre QC No. JRMDS-22-76279(PQ); Reviewed: 21-Oct-2022, QC No. JRMDS-22-76279; Revised: 05-Dec-2022, Manuscript No. JRMDS-22-76279(R); Published: 12-Dec-2022
