Research - (2022) Volume 10, Issue 6
Immunohistochemical Comparison of Peripheral and Central Giant Cell Granuloma Using Matrix Metalloproteinase-9 (Mmp-9)
Ghada Mohammed Mohmod* and Layla Sabri Yas
*Correspondence: Ghada Mohammed Mohmod, Department of Oral and maxillofacial pathology, Dental Teaching Hospital, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Introduction: Giant cell granulomas are confusing oral lesions distinguished by an unusual combination of mononuclear and multinucleated giant cells in fibroblastic vascularized background. Although central and peripheral giant cell lesions have the same histological features, their biological behavior was different. The objective the current study was aimed to assess a MMP-9 Immunoexpression in PGCG and CGCG, as well as the correlation between MMP-9 expression and their clinical behavior. Materials and Methods: thirty sample diagnosed as CGCG and PGCG were retrieved from the Pathology Laboratory at the College of the Dentistry/ University of Baghdad. The Immunohistochemical method was used to examine the expression of MMP-9 in CGCG and PGCG. Results: Matrix metalloproteinases-9 immunoreactivity was greater in the mononuclear cells of CGCL compared to PGCL. were similar in multinuclear giant cells of PGCL and CGCL. Conclusions: Matrix metalloproteinase-9 (MMP-9) expression was higher in CGCG than in PGCG, suggesting the variations in the clinical behavior related to protease overexpression.
Keywords
PGCG, CGCG, MMP-9, Immunohistochemistry
Introduction
Peripheral and central giant cell granuloma are two types of oral lesions defined by the presence of multinucleated giant cells within stroma of oval to spindle-shaped mononuclear cells. PGCG located in the soft tissues of the jaw, while CGCG is located intraosseously and has a tendency for expanding and destroying bone [1,2]. Peripheral giant cell granulomas develop due to a specific irritant factor or trauma, rarely erode the underlying bone, and have a relatively low risk of recurrence, particularly with proper management. But at the other hand, the origin of CGCG remains unknown, they are generally known to exhibit a range of clinical characteristics and behavior. Certain cases exhibit indolent behavior and few clinical features, whereas others manifest in a younger population, exhibit aggressive behavior, and have tendency to recurrence. Radiographic characteristics of aggressive lesions include tooth displacement, cortical perforation, and root resorption. Lesions that are not aggressive tend to have little or no symptoms and grow more slowly, accounting for most cases [3,4]. Despite histopathological similar, PGCL and CGCL behave differently clinically. Bone destruction occurs, most frequently in CGCL, to a minimal extent in PGCL cause cupping resorption [5]. The cells that degrade bones are termed osteoclasts, and certain proteins are essential for their activation and development [6]. Despite the reality that numerous studies have analyzed several aspects between, the causes underlying their different biologic behaviors remain unknown [7-9].
Past study has found that the activation of matrix- degrading enzymes such as matrix metalloproteinase (MMPs) appears to be involved in the balance between the creation and degradation of extracellular matrix (ECM) components seen in the diverse osteolytic lesions [10-12]. Although recent research shows that MMPs are produced by both multinucleated giant cells and the stromal cells in giant cell lesions [10,11]
MMPs are a group of zinc-dependent endopeptidases that can break down organic matrix in physiological pH conditions. MMPs have been associated with bone demineralization, and osteoblasts or osteoclasts can generate MMP9 [13]. MMP9 released by tumours can breakdown the basement membrane and extracellular matrix and can be marked to study endothelial cell migration and proliferation [10,14].
Furthermore, for many years, the pathophysiology of CGCG and PGCG in the jaws has been a point of controversy. Numerous studies have evaluated histopathological and Immunohistochemical characteristics in try to seek dependable markers associated with its distinct biological behavior. Additionally, results have demonstrated that the employment of quantitative approaches assists in the identification of latent aspects of diseases which may be missed during normal histopathological examinations However, due to there will be few studies examining the differences between two lesions, establishing a clear conclusion is problematic [7,15].
Unfortunately, no accurate markers exist to predict the prognosis or clinical behavior of CGGC and PGCG. The current study aims to evaluate the association between the Immunohistochemical expression of these protease and the clinical behavior of CGCG and PGCG, because there is evidence that MMP -9 may modulate bone resorption in pathological conditions.
Materials and Methods
The present study is conducted on thirty paraffin embedded block categorized as GCL, fifteenth of them CGCG and the other fifteen blocks belonged to PGCG, which were dated from 2013 to 2020. The archival samples were retrieved of the Department of oral and maxillofacial pathology/College of Dentistry/University of Baghdad. Demographic information, which included age, sex, and site was obtained from the surgical papers that received with the tissue specimens.
Four micrometer thick slices of the selected patients were cut from their archival paraffin blocks and treated regularly for histological and Immunohistochemical evaluation. Hematoxylin and eosin (H&E) were used to stain the sections, which were then viewed under a light microscope. In order to confirm a histopathological diagnosis. All cases were subjected to Immunohistochemical assessment and evaluation by biotin-avidin method. Antibody used (polyclonal Rabbit Anti-human MMP-9, Abcam ®, Cambridge, UK). Breast cancer was utilized as a positive control for the MMP-9 marker in this study.
The slides have been evaluated under a light microscope at magnification of 40x for MMP-9 cytoplasmic expression in MCs and MGCs (Figure 1). The presence of MMP-9 cytoplasmic staining was regarded positive. Immunohistochemical reactivity for MMP-9 was scored as follows, based on the percentage of positive cells in the mean average of five fields: score 0: no stained cells; score 1: ≤25% of cells were stained; score: 2 >25% and ≤50%; score 3: >50% and ≤75%; and score 4:>75% [16].
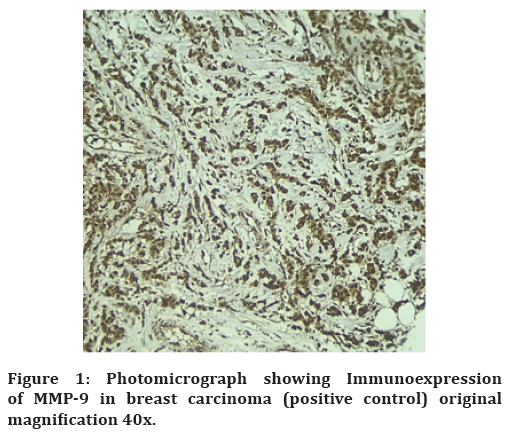
Figure 1. Photomicrograph showing Immunoexpression of MMP-9 in breast carcinoma (positive control) original magnification 40x.
Analyses were conducted statistically with the latest version of the Statistical Package for the Social Sciences (SPSS) 26. A descriptive and analytical study of the series was performed.
Results
The results of this study were examined in 15 PGCG and 15 CGCG cases during the time period indicated. The mean age (38.33±20.86) for central giant cell granulomas and (53± 13.58) for peripheral giant cell granuloma however, this was not a statistically significant difference (p = 0.05). Data regarding gender, the female predilection is more pronounced than the male in both PGCG and CGCG. In PGCG mandible was predominantly involved (11 cases) while only (4 cases) was found in maxilla. CGCG revealed a slight predisposition in the mandible (8 cases), later followed by the maxilla (7 cases), although the difference between the two lesions was not significant statistically (p = 0.5). As mentioned in Table 1.
| Characteristics | Study groups | ||
|---|---|---|---|
| CGCG Mean ± SD |
PGCG Mean ± SD |
P - Value | |
| Age (Years) | 38.33± 20.85 | 53±13.58 | 0.05 |
| Gender | |||
| Male | 7(46.7) | 4(26.7) | 0.5 |
| Female | 8(53.3) | 11(73.3) | |
| Site of Lesion | |||
| Maxilla | 7(46.7) | 4(26.7) | 0.5 |
| Mandible | 8(53.3) | 11(73.3) | |
| PGCG: peripheral giant cell granuloma CGCG: central giant cell granuloma |
|||
Table 1: The demographic data of patients with PGCG and CGCG.
The percentage of scores of MMP-9 positive giant cells and mononuclear cells in CGCG and PGCG (Figures 2 and 3). The percentage of MMP-9 expressions in multinucleated giant cells shown a score 4 (>75 percent positive cells) in both CGCG and PGCG. Regarding MMP-9 positivity in stromal cells the expression was higher in CGCG exhibited a predominance of score 4 in 15 (cases) However MMP-9 expression in stromal cells of PGCG was at score 4 in (9 cases) and score 3 in (5 cases) and score 2 in one case. In CGCL and PGCL, there is a significant different in immunoexpression of MMP-9 between multinucleated giant cells and stromal cells (p=0.00) as shown in Table 2.

Figure 2. MMP-9 immunostaining in giant cells and mononuclear cells of CGCG 40X.

Figure 3. MMP-9 immunostaining in giant cell and mononuclear cells of PGCG 40X.
| Marker | Cell type | Lesion subtype | Mann-Whitney U test | P-value | Sig. |
|---|---|---|---|---|---|
| MGC | C.G.C.G | 112.5 | 1 | NS | |
| MMP-9 | P.G.C.G | ||||
| C.G.C.G | 225 | 0 | S | ||
| MC | P.G.C.G | ||||
| MGC: Multinucleated giant cell MC: Mononuclear cell |
|||||
Table 2: MMP-9 expression levels were compared between giant cell lesions subtypes according to cell type.
There is no statistically significant difference in the expression of giant cells in two lesions (p=1.00) as shown in the Mann–Whitney test Median of MMP-9. As shown in Table 2, the only statistically significant difference in MMP-9 expression by stromal cells between CGCG and PGCG (p=0.00) was detected.
Discussion
This study compares proteolytic activity of MMP-9 in the two most commonly giant cell lesions of the jaw. CGCG shows predilection for young females above 30 years of age and the mandible is affected more than maxilla. while PGCG most prevalent in the fifth to sixth decades of life Predilection for females in agreement with previous studies published in the literature. Majority of these lesions are in the mandible, which was similarly shown in other studies based on published literature [17-19].
Furthermore, immunohistochemistry findings from in this study shows that giant cells markedly express the MMP-9 in CGCG and PGCG. Positive expression of MMP- 9 in the stromal mononuclear cells was higher in CGCL than in PGCL, in agreement with previous research indicating a key role for this endopeptidase is involved in the cleavage of demineralized organic substances of the bone matrix [9].
Matos et al. investigated MMP-9 immuno-expression in the CGCG and PGCG., MMP-9 expression was shown to be higher in CGCG. indicating that MMP-9 may be involved in the CGCG lesion osteoblastogenesis process Additionally, given the increased MMP-9 positivity in CGCG lesions in the current study, this marker may have a role in the osteoblastogenesis of CGCG lesions [11]. Rabinovich et al. found that MMP 9 overexpression in tumoral cells of giant cell tumors in long bone indicated that these cells are involved in the breakdown of stromal gelatin and bone invasions. MMP-9 was also generated by stromal and giant cells in the CGCG., suggesting that stromal cells and giant cells have a role in bone matrix degradation [13].
Also, the current research illustrates a series of immunohistochemistry evidence involving these two cellular components in order to establish much about how they communicate or influence one another. The existence of osteoclasts with MCs indicates that immunoinflammatory processes may be involved in the formation of these lesions. As a result, multinucleated giant cells recruiting, and induction are almost certainly the responsibility of mononuclear cell [9,20].
The findings of this study reveal that MMP-9 has a role in the resorptive activity in CGCG and PGCG, and that variations in the immunostaining of these proteolytic enzyme may relate to the clinical behavior differences.
Conclusion
The current findings of this study may help to explain why these two lesions have distinct clinical behaver despite their identical histopathologic features. Since MMP-9 expression of this marker more in CGCGs may be associated with a more aggressive clinical features, such as increased resorptive capacity and bone destruction compared to PGCGs.
References
- Neville B, Damm DD, Allen CM. Oral and maxillofacial pathology. 3rd Ed. Philadelphia: Saunders Elsevier, 2008: 626–629.
- Gupta S, Narwal A, Kamboj M, et al. Giant cell granulomas of jaws: A clinicopathologic study. J Oral Maxillofac Res 2019;10.
- Torres-Domingo S, Bagan JV, Jiménez Y, et al. Benign tumors of the oral mucosa: A study of 300 patients. Med Oral Patol Oral Cir Bucal 2008; 13:E161-E166.
- Sadri D, Hejazi M, Jahanbani J, et al. Quantitative analysis of argyrophilic nuclear organizer regions in giant cell lesions of jaws. J Oral Pathol Med 2010; 39:431-434.
- Souza PE, Mesquita RA, Gomez RS. Evaluation of p53, PCNA, Ki‐67, MDM2 and AgNOR in oral peripheral and central giant cell lesions. Oral Dis 2000; 6:35-39.
- Engsig MT, Chen QJ, Vu TH, et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J Cell Biol 2000; 151:879-890.
- Torabinia N, Razavi SM, Shokrolahi Z. A comparative immunohistochemical evaluation of CD68 and TRAP protein expression in central and peripheral giant cell granulomas of the jaws. J Oral Pathol Med 2011; 40:334-337.
- Pires Duarte A, Cavaliéri Gomes C, Santiago Gomez R, et al. Increased expression of NFATc1 in giant cell lesions of the jaws, cherubism and brown tumor of hyperparathyroidism. Oncol Lett 2011; 2:571-573.
- Liu B, Yu SF, Li TJ. Multinucleated giant cells in various forms of giant cell containing lesions of the jaws express features of osteoclasts. J Oral Pathol Med 2003; 32:367-375.
- Kumta SM, Huang L, Cheng YY, et al. Expression of VEGF and MMP-9 in giant cell tumor of bone and other osteolytic lesions. Life Sci 2003; 73:1427-1436.
- Cowan RW, Mak IW, Colterjohn N, et al. Collagenase expression and activity in the stromal cells from giant cell tumour of bone. Bone 2009; 44:865-871.
- Mak IW, Cowan RW, Popovic S, et al. Upregulation of MMP-13 via Runx2 in the stromal cell of Giant Cell Tumor of bone. Bone 2009; 45:377-386.
- Kusano K, Miyaura C, Inada M, et al. Regulation of matrix metalloproteinases (MMP-2,-3,-9, and-13) by interleukin-1 and interleukin-6 in mouse calvaria: Association of MMP induction with bone resorption. Endocrinology 1998; 139:1338-1345.
- Rabinovich A, Mak IW, Cowan RW, et al. Matrix metalloproteinase activity in the stromal cell of giant cell tumor of bone. Open Bone J 2009; 1:46-52.
- Flórez-Moreno GA, Henao-Ruiz M, Santa-Sáenz DM, et al. Cytomorphometric and immunohistochemical comparison between central and peripheral giant cell lesions of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 2008; 105:625-632.
- Mohtasham N, Anvari K, Memar B, et al. Expression of E-cadherin and matrix metalloproteinase-9 in oral squamous cell carcinoma and histologically negative surgical margins and association with clinicopathological parameters. Rom J Morphol Embryol 2014; 55:117-121.
- Sun ZJ, Cai Y, Zwahlen RA, et al. Central giant cell granuloma of the jaws: Clinical and radiological evaluation of 22 cases. Skeletal Radiol 2009; 38:903-909.
- Aghbali A, Sina M, Pakdel SM, et al. Correlation of histopathologic features with demographic, gross and radiographic findings in giant cell granulomas of the jaws. J Dent Res Dent Clin Dent Prospects 2013; 7:225.
- Katsikeris N, Kakarantza-Angelopoulou E, Angelopoulos AP. Peripheral giant cell granuloma. clinicopathologic study of 224 new cases and review of 956 reported cases. Int J Oral Maxillofac Surg 1988; 17:94-99.
- Syrio NF, Faria DR, Gomez RS, et al. IL-10 and IL-10 receptor overexpression in oral giant cell lesions. Med Oral Patol Oral Cir Bucal 2011; 16:e488-492.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Ghada Mohammed Mohmod* and Layla Sabri Yas
Department of Oral and maxillofacial pathology, Dental Teaching Hospital, College of Dentistry, University of Baghdad, IraqReceived: 02-May-2022, Manuscript No. JRMDS-22-62392; , Pre QC No. JRMDS-22-62392 (PQ); Editor assigned: 04-May-2022, Pre QC No. JRMDS-22-62392 (PQ); Reviewed: 19-May-2022, QC No. JRMDS-22-62392; Revised: 26-May-2022, Manuscript No. JRMDS-22-62392 (R); Published: 02-Jun-2022
