Research Article - (2022) Volume 10, Issue 4
Immunohistochemical Evaluation of HPV, Proliferation and Apoptosis in Oral Squamous Cell Carcinoma among Young and Old Patients: Comparative Study
*Correspondence: Alaa S Saeed, Department of Oral Diagnosis, College of Dentistry, University of Baghdad, Iraq, Email:
Abstract
Objectives: Oral Squamous Cell Carcinoma is a disease of adults that rarely develops before the age of fifty. However, there is a gradual increase in the occurrence of the disease among young people over the world. As reported in many publications, a tumor developing in young patients lacks the usual associated risk factors, such as tobacco smoking, and thus such a tumor has an aggressive outcome. This study aims to compare the tumor development among young and old patients in terms of etiological and biological behavior using immunohistochemistry for the high-risk human papilloma virus, proliferative and apoptotic markers. Material and methods: This study was conducted on 35 cases of paraffin-embedded tissue blocks of oral squamous cell carcinoma divided into two age groups, twenty cases>40 years, and fifteen cases ≤40 years. The Clinocopathological finding was collected and recorded. Immunohistochemical analysis was performed using HPV 16/18, p16, p21, ki-67and p53. Results: This study did not reveal a significant difference between the tumor of the young and old patients regarding the viral receptors, proliferation, and apoptosis. Where p-value >0. 05. Conclusion: Viral expression, proliferation, and apoptosis have no effect on tumor differentiation among young and old patients.
Keywords
Oral squamous cell carcinoma, HPV, p16, p21, ki-67, p53, age group
Introduction
Oral Squamous Cell Carcinoma (OSCC) is an epithelial malignancy that commonly metastasis for the adjacent lymph node. Even though the incidence of this malignancy is rare in young individuals ranging from 1 % to 6 % [1], however its rate has been increased in the last few years [2]. Developing of this malignancy in young patients is mostly associated with different etiology, different prognosis, and biological behavior [1]. The potentially attributing risk factors in young patients may include viruses, especially a high-risk human papillomavirus HPV, genetic predisposition, feeding habits, and immunosuppressive conditions [3]. The tumor appears more aggressive in this subset of patients, so many research and studies have been made to define the nature of the aggressiveness of this malignancy, and the possible associated risk factors using a different molecular markers [4]. As previous studies suggest high risk HPV, especially HPV 16 and 18, may play a role in oral carcinogenesis, particularly in young individuals [5,6]. Research reported that the HPV can promote tumorigenesis by transforming the infected epithelial cells in to malignant cells, and may cause a defect in the genes that control the apoptosis, cell cycle, and DNA repair [7]. Many studies pointed to the role of p16, which is a tumor suppressor gene in OSCC. Studies suggested, a high expression rate for p16 in HPV infected tumor due to the transforming activity of E7.
It’s well known that one of the significant biological mechanisms in oncogenesis is the disturbance in cells proliferation. Ki-67 is an important protein in cell cycle regulation and can be used to assess the growth fraction [8]. Cell cycle proteins dysregulation is evident in oral cancers , hence it can play a role in OSCC biological behavior [9].
P53 is a molecular marker that can be detected immunohistochemically in a nearly half of all oral cancers, and it is one of the most remarkable cell cycle regulatory proteins ,where its activation can prompt the growth arrest, as well as the cell death. However, when this gene gets mutated, it will lose its function, hens its regulatory action on normal cell cycle control and cell proliferation will inhibit [10,11]. P21 protein, which is a cyclindependent kinase inhibitor regulated by p53-dependent and independent pathways is frequently expressed in OSCC [12].
Hypothetically, different molecular mechanisms may be seen in OSCC of young versus old patients; accordingly, different patterns of cell cycle proteins expression may be seen. This study intended to compare the expression of HPV, proliferative and apoptotic markers in OSCC from patients above and below the age of 40, and correlate the expression with the Clinocopathological characters including the tumor staging and grading using the following markers (HPV E6/16,18, p16, Ki-67, p53, p21). It is worth mentioning that young age categorization is difficult to establish, however most of the studies used the age of 40 as a cut-off point between young and old patients [13,14].
Materials and Methods
This study was conducted on 35case of formalin-fixed paraffin-embedded tissue blocks of OSCC. From each case, five sections of a four-micron thickness were subjected to standard immunohistochemical protocol using a Leica staining kit. The cases were divided into two age groups including 20 cases with patients >40 and 15 ≤ 40. Two investigators evaluated the staining. The scoring was considered positive according to the following scale: score 1: 10-25% cells score 2:25-50% cells score 3: 50- 75% cells, Score 4: >75% cells positive. Both intensity and proportion have been evaluated, where the intensity is graded as a weak, moderate, and strong. For the statistical analysis of the data, multivariate analysis was used for evaluating the expression of each protein in relation to other variables.
Results
This study did not reveal significant association between the markers expression and the Clinocopathological parameters including: gender of the patient, tumor anatomic site, perineural invasion, tumor grading and staging where p-value > 0.05 (Figure1).
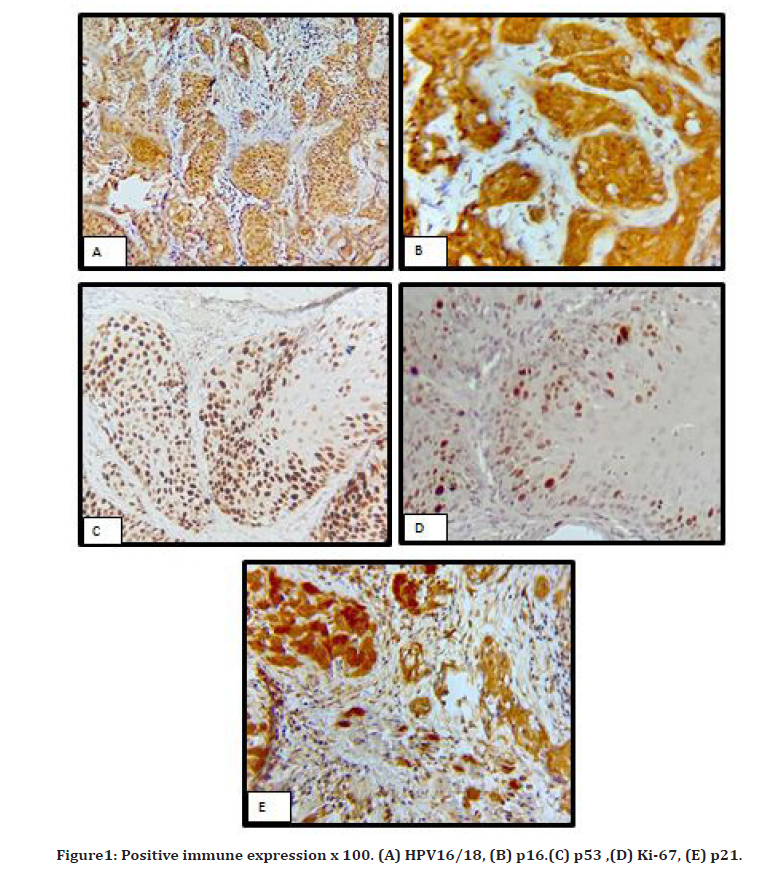
Figure 1. Positive immune expression x 100. (A) HPV16/18, (B) p16.(C) p53 ,(D) Ki-67, (E) p21.
However, the rate of positive expression for p53, p21, ki-67 were significantly higher in elderly than younger patients where p-value <0.05 (Tables 1-3).
| Variables | HPV | P16 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive No | Negative No | OR | P. Value | Positive No | Negative No | OR | P. Value | ||
| Age (year) | = 40 | 2 | 13 | 0.697 | 0.095 | 4 | 11 | 0.951 | 0.814 |
| > 40 | 8 | 12 | 6 | 14 | |||||
| Gender | Male | 5 | 12 | 1.117 | 0.599 | 6 | 11 | 1.214 | 0.377 |
| Female | 5 | 13 | 4 | 14 | |||||
| Site | Tongue | 4 | 18 | 0.757 | 0.164 | 5 | 17 | 0.866 | 0.476 |
| Floor | 3 | 3 | 2 | 4 | |||||
| Buccal mucosa | 2 | 1 | 2 | 1 | |||||
| Other | 1 | 3 | 1 | 3 | |||||
| Grade | Low grade | 6 | 7 | 1.062 | 0.83 | 5 | 8 | 0.969 | 0.914 |
| Intermediate grade | 3 | 11 | 4 | 10 | |||||
| High grade | 1 | 7 | 1 | 7 | |||||
| Stage | Stage 1-2 | 5 | 6 | 0.981 | 0.94 | 5 | 6 | 1.015 | 0.955 |
| Stage 3-4 | 4 | 13 | 4 | 13 | |||||
| Perineural Invasion | Positive | 2 | 6 | 0.825 | 0.477 | 0 | 8 | 1.155 | 0.127 |
| Negative | 8 | 19 | 10 | 17 | |||||
Table 1: Multivariate analysis of HPV and p16 in relation to other variables
| Variables | p53 | p21 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive No | Negative No | OR | P. Value | Positive | Negative | OR | P. Value | ||
| Age (year) | = 40 | 6 | 9 | 0.64 | 0.041 | 6 | 9 | 0.574 | 0.004 |
| > 40 | 15 | 5 | 13 | 7 | |||||
| Gender | Male | 11 | 6 | 1.287 | 0.235 | 10 | 7 | 1.224 | 0.257 |
| Female | 10 | 8 | 9 | 9 | |||||
| Site | Tongue | 15 | 7 | 1.194 | 0.365 | 10 | 12 | 0.764 | 0.108 |
| Floor | 3 | 3 | 4 | 2 | |||||
| Buccal mucosa | 2 | 1 | 3 | 0 | |||||
| Other | 1 | 3 | 2 | 2 | |||||
| Grade | Low grade | 8 | 5 | 0.822 | 0.486 | 7 | 6 | 1.15 | 0.553 |
| Intermediate grade | 8 | 6 | 8 | 6 | |||||
| High grade | 5 | 3 | 4 | 4 | |||||
| Stage | Stage 1-2 | 7 | 4 | 0.786 | 0.346 | 5 | 6 | 0.511 | 0.004 |
| Stage 3-4 | 11 | 6 | 13 | 4 | |||||
| Perineural Invasion | Positive | 5 | 3 | 0.656 | 0.126 | 6 | 2 | 0.84 | 0.44 |
| Buccal mucosa | 16 | 11 | 13 | 14 | |||||
Table 2: Multivariate analysis of p53and p21 in relation to other variables
| Variables | Ki-67 | ||||
|---|---|---|---|---|---|
| Positive No | Negative No | OR | P. Value | ||
| Age (year) | = 40 | 7 | 8 | 0.595 | 0.007 |
| >40 | 18 | 2 | |||
| Gender | Male | 13 | 4 | 1.221 | 0.309 |
| Female | 12 | 6 | |||
| Site | Tongue | 16 | 6 | 0.919 | 0.638 |
| Floor | 4 | 2 | |||
| Buccal mucosa | 3 | 0 | |||
| Other | 2 | 2 | |||
| Grade | Low grade | 10 | 3 | 1.063 | 0.813 |
| Intermediate grade | 11 | 3 | |||
| High grade | 4 | 4 | |||
| Stage | Stage 1-2 | 9 | 2 | 0.836 | 0.45 |
| Stage 3-4 | 11 | 6 | |||
| Perineural Invasion | Positive | 5 | 3 | 0.749 | 0.251 |
| Negative | 20 | 7 | |||
Table 3: Multivariate analysis of Ki-67 in relation to other variables
Discussion
High-risk human papillomaviruses have an important role in the etiology of OSCC, however the literatures vary in the prevalence rate ranging from 20-50% , and this variation may be related to the technique of identification, the geographic or the ethnic distribution, the sample size, the method of preparation, whether it is formalinfixed or fresh frozen , and the tumor anatomical site [15]. The present study did not reveal significant difference between HPV expression and the Clinocopathological parameters of the compared groups , which was in agreement with other studies [15-18]. HPV can induce carcinogenesis alone or with other well-known risk factors including tobacco and/or alcohol. The present study did not show a significant difference between p16 expression and the clinicopathological parameters. This is again in agreement with other studies [19-21]. The relation between HPV and p16 is still a matter of discussion. Many authors mentioned that overexpression of p16INK4A protein by immunohistochemistry can act as a biomarker for HPV-induced oral carcinomas [21,22]. The current study revealed a significant correlation between HPV and p16 INK4A. However, the studies were variable regarding this aspect, and this variation may be related to variation in methodologies or the techniques that were used for HPV and p16 identification ,in addition to variation in the scoring system, or variation in the types of HPV that were used in the studies [23].
There are many regulators for the cell cycle such as: cyclins, cyclin dependent kinases, oncogenes, and tumor suppressor genes. These regulators play a crucial role in the cell cycle maintenance, and control the balance between growth and death of the cell. One of these important regulators is p21 [24]. According to many literatures this new emerging marker has a valuable role in predicting the prognosis of HNSCC. The current study showed a significant correlation for p21 expression and age category on multivariate analysis. Where p21 is highly expressed in adult patients. This is in agreement with other studies [12,25]. The present study did not show any significant correlation for p21 expression and other Clinocopathological parameters in both age groups [26]. In a fact, there are not many previous reports on the study of p21 expression in OSCC, and most of the literatures studied the gene expression in the tumor from different sites of the oral cavity. Tumors developing at a different site of the head and neck region have different biologic behaviors, so the clinical features, risks of lymphatic invasion, treatment modalities, and prognoses are highly variable [25].
Regarding the proliferative aspect, this study did not reveal a significant correlation for the proliferative marker and the Clinocopathological parameters of the compared groups, however the rate of expression was significantly higher in young than old patients, which was in agreement with other studies [8,27]. The role of ki-67 in evaluating the outcome of OSCC is still vague, and finding from the previous studies were conflicting. This may be due to variation in the methods used to assess Ki-67expression, or variations in the process applied for counting the positive tumor cells among other cells.
Concerning apoptosis, the current study did not show a significant difference in p53 expression and the Clinocopathological parameters in both age groups, however the expression rate was higher in old patients. This finding is similar to other studies [28,29]. The high apoptotic rate in the old patients could be attributed to many causes such as: a prolonged duration of carcinogen exposure with a low immune response, or delay in a tumor diagnosis. The result of the study about p53 expression and its relation to other parameters is highly variable, and affected by different parameters such as techniques that were used, and the sample size.
Conclusion
Proliferative and replicative potential, apoptosis, and viral expression did not show any significant effect in determining the biological behavior of OSCC considering the age groups, namely young vs. elderly.
Ethical Approval
All experimental protocols were approved by the College of Dentistry, University of Baghdad. All experiments were carried out following the approved guidelines. (Ref no.167719 on 31/12/2019).
Financial Support
There was no financial disclosure.
Conflict of Interest
The authors declare that there are no conflicts of interest.
References
- Ribeiro AC, Silva AR, Simonato LE, et al. Clinical and histopathological analysis of oral squamous cell carcinoma in young people: A descriptive study in Brazilians. Br J Oral Maxillofac Surg 2009; 47:95-8.
- Sun Q, Fang Q, Guo S. A comparison of oral squamous cell carcinoma between young and old patients in a single medical center in China. Int J Clin Exp Med 2015; 8:12418.
- Llewellyn CD, Johnson NW, Warnakulasuriya KA. Risk factors for squamous cell carcinoma of the oral cavity in young peopleâ??A comprehensive literature review. Oral Oncol 2001; 37:401-18.
- Gonzalez-Moles MA, Ruiz-Avila I, Gil-Montoya JA, et al. Analysis of Ki-67 expression in oral squamous cell carcinoma: why Ki-67 is not a prognostic indicator. Oral Oncol 2010; 46:525-30.
- Chocolatewala NM, Chaturvedi P. Role of human papilloma virus in the oral carcinogenesis: an Indian perspective. J Cancer Res Ther 2009; 5:71.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294.
- Machado J, Reis PP, Zhang T, et al. Low prevalence of human papillomavirus in oral cavity carcinomas. Head Neck Oncol 2010; 2:1-6.
- Gontarz M, Wyszyn P. G, Zapaa J. Immunohistochemical predictors in squamous cell carcinoma of the tongue and floor of the mouth. Head Neck 2016; 38:E747-53.
- Scully C, Bagan JV. Oral squamous cell carcinoma: overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis 2009; 15:388-99.
- Kuroda Y, Nakao H, Ikemura K, et al. Association between the TP53 codon72 polymorphism and oral cancer risk and prognosis. Oral Oncol 2007; 43:1043-8.
- Nylander K, Dabelsteen E, Hall PA. The p53 molecule and its prognostic role in squamous cell carcinomas of the head and neck. J Oral Pathol Med 2000; 29:413-25.
- Ng IO, Lam KY, Ng M, et al. Expression of p21/waf1 in oral squamous cell carcinomasâ??correlation with p53 and mdm2 and cellular proliferation index. Oral Oncol 1999; 35:63-9.
- Müller S, Pan Y, Li R, et al. Changing trends in oral squamous cell carcinoma with particular reference to young patients: 1971â??2006. The Emory University experience. Head Neck Pathol 2008; 2:60-6.
- Komolmalai N, Chuachamsai S, Tantiwipawin S, et al. Ten-year analysis of oral cancer focusing on young people in northern Thailand. J Oral Sci 2015; 57:327-34.
- Sreejyothi H.K, Harishchandra R, Shreedevi B. Role of Human Papilloma Virus in Oral Squamous Cell Carcinoma. Int J Contemp Med 2017; 4.
- Elango KJ, Suresh A, Subhadradevi L, et al. Role of human papilloma virus in oral tongue squamous cell carcinoma. Asian Pac J Cancer Prev 2011; 12:889-96.
- Jalal H, Sanders CM, Prime SS, et al. Detection of human papilloma virus type 16 DNA in oral squames from normal young adults. J Oral Pathol Med 1992; 21:465-70.
- Blahak J, Zelinka J, Gumulec J, et al. HPV, protein p16 and squamous cell carcinoma of the oral cavity. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2020; 164.
- Ralli M, Singh S, Yadav SP, et al. Assessment and clinicopathological correlation of p16 expression in head and neck squamous cell carcinoma. J Cancer Res Ther 2016; 12:232.
- Barnabé L�, Batista AC, MENDON�A EF et al. Cell cycle markers and apoptotic proteins in oral tongue squamous cell carcinoma in young and elderly patients. Braz Oral Res 2019; 33.
- Gröbe A, Hanken H, Kluwe L et al. Immunohistochemical analysis of p16 expression, HPV infection and its prognostic utility in oral squamous cell carcinoma. J Oral Pathol Med 2013; 42:676-81.
- Gabrielli Fregonesi PA, Teresa DB, Duarte RA, et al. p16INK4A immunohistochemical overexpression in premalignant and malignant oral lesions infected with human papillomavirus. J Histochem Cytochem 2003; 51:1291-7.
- Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in United States cooperative group trials. Am J Surg Pathol 2012; 36:945.
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9:400-14.
- Regezi JA, Dekker NP, McMillan A, et al. p53, p21, Rb, and MDM2 proteins in tongue carcinoma from patients< 35 versus> 75 years. Oral Oncol 1999; 35:379-83.
- Fischer CA, Jung M, Zlobec I, et al. Co-overexpression of p21 and Ki-67 in head and neck squamous cell carcinoma relative to a significantly poor prognosis. Head Neck 2011; 33:267-73.
- de Proliferación Celular ID. Immunoexpression of cell proliferation markers in oral squamous cell carcinoma. Int J Odontostomat 2016; 10:513-520.
- Chandra A, Singh A, Sebastian BT, et al. Oral squamous cell carcinomas in age distinct population: A comparison of p53 immunoexpression. J Cancer Res Ther 2013; 9:587.
- Rushatamukayanunt P, Morita KI, Matsukawa S, et al. Lack of association between high-risk human papillomaviruses and oral squamous cell carcinoma in young Japanese patients. Asian Pac J Cancer Prev 2014; 15:4135-41.
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Author Info
1Department of Oral Diagnosis, College of Dentistry, University of Baghdad, IraqReceived: 07-Mar-2022, Manuscript No. JRMDS-22-51580; , Pre QC No. JRMDS-22-51580 (PQ); Editor assigned: 09-Mar-2022, Pre QC No. JRMDS-22-51580 (PQ); Reviewed: 23-Mar-2022, QC No. JRMDS-22-51580; Revised: 28-Mar-2022, Manuscript No. JRMDS-22-51580 (R); Published: 04-Apr-2022
