Research - (2020) Advances in Dental Surgery
Incidence and Antibiotic Sensitivity of Anaerobic Bacteria in Dentoalveolar Abscess of Diabetic and Non-Diabetic Patients: A Cross Sectional Comparative Study
*Correspondence: Tejashree R, Department of Dentistry, Bowring Lady Curzon Medical College and Research Institute, Bangalore, Karnataka, India, Email:
Abstract
Background: The presentation of bacterial infection of dental origin is constantly changing and is a measurable reflection of modern evolution of oral flora, the poor host response is multifactorial and diabetes has long been considered as one of the factors reducing host response. Antibiotic resistance patterns may change over time, in Diabetic patient’s frequent use of broad-spectrum antibiotics in the treatment of oral infections and other infections may enhance the development of bacterial resistance. Aim is to compare the incidence, Antibiotic sensitivity of anaerobic isolates from Dentoalvelar abscess of Non-Diabetic and Diabetics. Method: This descriptive study was conducted on 120 patients, both Diabetic (60) and Non-Diabetic (60) patients with Dentoalveolar abscess. Purposive sampling was done. The pus sample collected; cultured (aerobically and anaerobically). Morphological, biochemical, and antibiotic sensitivity tests were done to study the isolates. Statistical analysis was done using unpaired Student’s t test, Chi-square, or Fisher’s exact test, found significant if p <0.05. Results: Anaerobic isolates from Diabetic patients were significantly high, majority were Gram negative isolates. Antibiotic sensitivity was comparable with respect to Metronidazole most sensitive (100%) followed by Amoxicillin/Clavulanic acid (90%). Isolates from Diabetic patients were significantly resistant to Ciprofloxacin (p<0.0001). Conclusions: Anaerobic bacteria are more prevalent in Diabetic patients, anaerobes are subjected throughout life to a continuous challenge by antimicrobial agents used in clinical therapy, high prevalence of bacterial resistance to Ciprofloxacin, Ampicillin suggests the need for regular antibiotic susceptibility tests and rational use of antibiotics in the management of these infections.
Keywords
Diabetes Mellitus, Abscess, Bacterial Infections, Metronidazole
Introduction
Dental or Dentoalveolar abscess is a denomination used to describe localized collection of pus in the alveolar bone at the root apex of the tooth. It usually occurs secondary to dental caries, trauma, deep fillings, or failed root canal treatment. The pathogenesis of Dentoalveolar abscess is polymicrobial in nature, comprising of various facultative anaerobes, such as the viridans group streptococci and the Streptococcus anginosus group, and strict anaerobes, especially anaerobic cocci, Prevotella and Fusobacterium species [1]. These microorganisms can form biofilms in root canals, hence making application of the “biofilm concept” plausible in such infections. Biofilms form not only within the root canal, but also on the root surface, in the apical region [2]. The treatment of orofacial infections is part of an everyday practice in Dentistry. Odontogenic abscess-forming infection usually spreads into the potential anatomical spaces present in the oral and maxillofacial region. The area of least resistance usually governs the spread with the host defense mechanism and virulence of the organism playing an important role as well [3].
The poor host response is multifactorial, and diabetes has long been considered as one of the factors reducing host response. One of the serious complications of diabetes includes predisposition to infections. Diabetic individuals are not only at high risk for infectious disease, but it is also believed that they often respond poorly to infections once they occur [4].
The presentation of bacterial infection of dental origin is constantly changing and is a measurable reflection of modern evolution of oral flora [5]. It is not only the host defense that determines the outcome of infection, but the timing and appropriateness of antimicrobial treatment as well [6]. As bacterial pathogens and antibiotic resistance patterns may change over time and based on location, in diabetic patients, the selfmedication and frequent use of broad spectrum antibiotics in the treatment of oral infections and other body infections may enhance the development of bacterial resistance [7]. Our study was designed with an objective to compare the role of anaerobic bacteria in Dentoalveolar abscess in Diabetic patients with Non-Diabetics by isolating and identify anaerobic organisms and testing their antibiotic sensitivity in order to provide guidelines for effective treatment.
Subjects and Methods
This cross-sectional, comparative study was conducted for 1 year from January 2019 to December 2019 after obtaining institutional ethical clearance; a total of 120 patients with acute Dentoalveolar abscess who visited the outpatient department of oral medicine and radiology were included in the study and divided into 2 groups:
Group I (Non-Diabetic group) thirty patients were included in the group who had normal blood glucose levels at the time of reporting, no history of Diabetes.
Group II (Diabetic group) thirty patients who had fasting blood glucose level more than 130 mg/dL (7.2 mmol/L) or had a known history of Diabetes but had controlled sugar levels were also included in the Diabetic group.
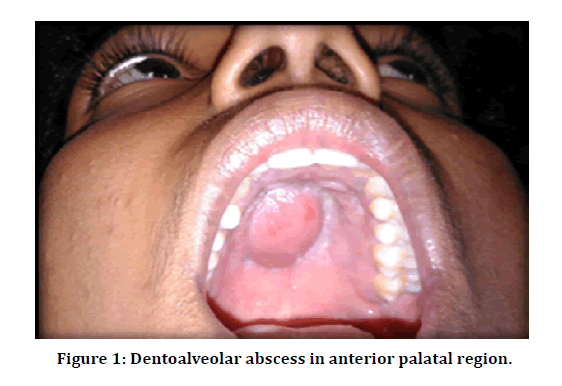
Purposive sampling was done; we included patients aged between 40 to 65 years with dent alveolar abscess with intraoral or extra oral swelling, (Figure 1) we excluded patients with previous endodontic therapy of the affected tooth, teeth with periapical sinus/ fistula, antibiotic therapy with in previous two months. A brief case history along with Diabetic history taken and investigated for blood HbA1c levels, if clinical findings satisfied the inclusion criteria, the patient was informed about all the procedures to be performed during the study. Following that, if patient was ready to be a part of the study, the patient was asked to sign the consent form.
Figure 1: Dentoalveolar abscess in anterior palatal region.
Procedure was explained to the patient. Patient was seated on a dental chair and draped with a patient drape. Patient was asked to rinse mouth with water. For each patient, the oral mucosa overlying the abscess was scrubbed with tincture of iodine; a sterile 18- gauge needle fitted to a 3-ml disposable syringe was passed through the alveolar mucosa into the abscess, from which the contents are withdrawn. The needle was sealed immediately by cork. The samples are transported to microbiology laboratory held at room temperature and processed within 30 mins.
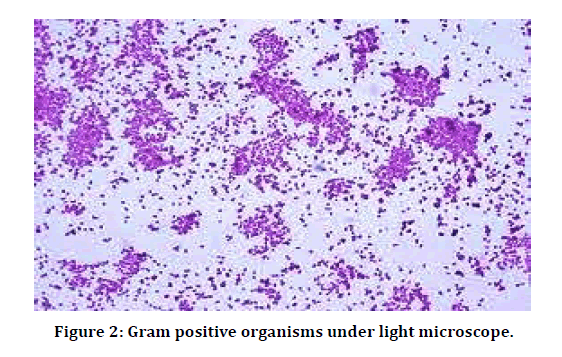
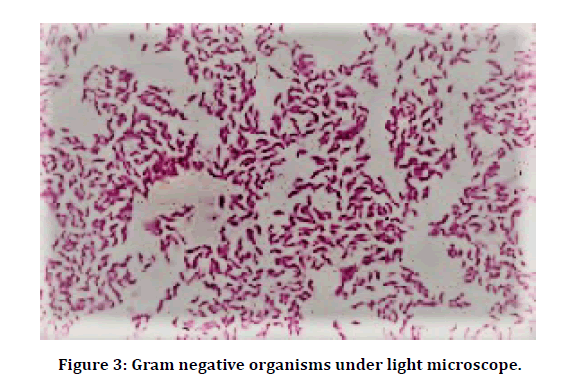
Sample processing was done in the following way: Direct microscopy for Bacterial morphology: A thin smear of the pus sample was made on a clean glass slide and allowed to air dry. Smear was gently heat fixed, stained by Gram staining technique, and examined under oil immersion objective of the light microscope, for the presence of pus cells, Gram positive (Figure 2) and Gram negative (Figure 3) organisms. The size, shape and arrangement of bacteria were noted.
Figure 2: Gram positive organisms under light microscope.
Figure 3: Gram negative organisms under light microscope.
Anaerobic culture: The sample was inoculated onto Brucella blood agar containing vitamin K and Hemin in which a Metronidazole disc was placed at the junction of primary and secondary streaking to identify the anaerobes presumptively. A MacConkey agar plate streaked with Pseudomonas aeruginosa ATCC strain which is an obligate aerobe was used as a negative control for anaerobiosis. The plates were immediately incubated anaerobically for 48 hours at 37°C in an anaerobic jar (Figure 4) (Hi media Anaerobic System Mark II LE 002 3.5L) with Gaspak (BD GasPak EZ Gas Generating Container Systems with indicator).
Figure 4: Anaerobic jar with plates and Gaspak.
After 48hrs plates were examined for growth and colony morphology of different colonies were recorded Transmitted light was used to look for hemolysis. Gram stained smears of the colonies were examined under oil immersion objective of the microscope. Catalase and Spot indole test were performed. Each colony were then subculture onto brucella blood agar plates and based upon gram staining Nitrate disk, Bile esculin disk and Special potency disk were used for gram negative, Sodium polyanethol sulphonate (SPS) disk was used for gram positive and the plates were incubated in the anaerobic jar with Gaspak at 37°C for 48 hrs. Aerotolerance test was done to differentiate between obligate and facultative anaerobes. Zone of inhibition were measured after incubation and results were interpreted. Biochemical tests (catalase, Nitrate disk reduction test, coagulase, Bile test, Spot indole test, Sodium polyanethol sulphonate test, Special potency antibiotic disc susceptibility) were performed.
Antimicrobial susceptibility testing was performed on anaerobic isolates, by disc diffusion method. Colonies of bacteria are spread over Muellerâ??Hinton agar medium. Discs impregnated with antibiotics are placed by the help of sterilized forceps. This plate was again incubated for 12–24 h at 37°C. Zone of inhibition is measured by the help of the WHO quality control chart to access the sensitivity of the antibiotics (Amoxicillin, Amoxicillin &clavulanic acid, Clindamycin, Cefotaxime, Metronidazole, Ciprofloxacin, antibiotics were selected for testing).
Statistical analysis
Data are expressed as means ± SD or percentages. To compare continuous variables, we used the unpaired Student’s t test, Mann Whitney U test and Wilcoxon test. For categorical variables, we applied the Chi-square or Fisher’s exact test. Comparison of proportions was performed using the Fisher’s exact test. “P value” is non-significant if >0.05 and significant if <0.05. The Statistical software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment ver.2.11.1 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs, tables etc.
Results
The study population comprised of 120 patients, 60 each in Non-Diabetic and Diabetic group, demographic parameters with respect to age and weight (Table 1) comparable between the groups. Sex distribution (Table 2) for patients from both Diabetic and non-Diabetic groups is shown in Table 2 which is a total of 56 (46.6%) males, and a total of 64 (53.3%) females; there are no significant differences (p value 0.0992) between the two groupsNon-Diabetic group had 90% and Diabetic group had 95% positive cultures, (Table 3) Cultures in Diabetic group had significantly more isolates per culture (Table 3) Gram Positive isolates are more common in Non-Diabetic group and Gram Negative isolates common in Diabetic group. (Table 3) Common isolates among both groups are Peptostreptococcus, Peptococccus, Fusobacterium, Prevotella, and Bacteroide (Table 4).
| Parameters | Non-Diabetic | Diabetic | p value |
|---|---|---|---|
| NUMBER | 60 | 60 | |
| Age (mean ± sd) | 58 ± 12 | 61 ± 8 | 0.109 |
| Weight | 72.6 ± 14.6 | 78.3 18.5 | 0.0635 |
| Average isolates per patient | 1.533 | 1.95 | <0.0001* |
*Statistically significant
Table 1: Demographic data.
| Gender | Patients | Non- Diabetic | Diabetic |
|---|---|---|---|
| Male | 56 | 33 (55%) | 23 (38.33%) |
| Female | 64 | 27 (45%) | 37 (61.66%) |
Table 2: Numbers and percentages of non-diabetic and diabetic patients based on sex.
| Culture findings | Non-Diabetic | Diabetic | p-Value | ||
|---|---|---|---|---|---|
| Specimens(n) | Percentage (%) | Specimens(n) | Percentage (%) | ||
| Positive cultures | 54 | 90% | 57 | 95% | 0.3 |
| Negative cultures | 6 | 10% | 3 | 5% | 0.3 |
| 1 isolate/ Culture | 16 | 26% | 9 | 15.78% | 0.17 |
| 2 isolates/ Culture | 38 | 60% | 48 | 84.21% | 0.003* |
| Total isolates | 92 | 116 | 0.0961 | ||
| Gram Positive | 62 | 67% | 57 | 49.13% | 0.009* |
| Gram Negative | 30 | 33% | 59 | 50.86% | 0.009* |
n-Total number *statistically significant
Table 3: Culture results.
| Total Isolates | Nondiabetic | Diabetic | p value | ||
|---|---|---|---|---|---|
| Isolates (n) | Percentage (%) | Isolates(n) | Percentage (%) | ||
| Gram Positive | |||||
| Peptostreptococcus | 33 | 35.86 | 31 | 26.72 | 0.157 |
| Peptococccus | 29 | 31.52 | 26 | 22.41 | 0.14 |
| Gram Negative | |||||
| Fusobacterium | 16 | 17.4 | 29 | 25 | 0.187 |
| Prevotella | 8 | 8.7 | 19 | 16.37 | 0.102 |
| Bacteroides | 6 | 6.52 | 11 | 9.48 | 0.44 |
n- Total number of Isolates
Table 4: Distribution of bacterial isolates in Non-Diabetic and diabetic patients.
Isolates form both the groups are 100% sensitive to Metronidazole (Table 5) and both groups are 100% resistant to amoxicillin, isolates from Diabetic group are more resistant (p<0.0001) to Ciprofloxacin.
| Drug | Non-Diabetic | Diabetic | P-Value |
|---|---|---|---|
| Metronidazole | 100% | 100% | 0 |
| Amoxicillin+Clavulanic acid | 90% | 95% | 0.167 |
| Clindamycin | 85% | 83% | 0.697 |
| Ciprofloxacin | 90% | 60% | <0.0001* |
| Cefotaxime | 75% | 72% | 0.627 |
*Statistically significant
Table 5: Comparison of Antibiotic sensitivity of isolates from both the groups.
Discussion
Major advances have occurred in the field of bacterial culture and identification techniques, due to which predominantly anaerobes have been found in orofacial infections particularly gramnegative microflora [8]. Major limitation of past cultural studies is that a large percentage of the oral microflora does not grow on conventional artificial culture media in the laboratory [9]. The early investigation on dental periapical abscesses failed to utilize adequate anaerobic culture techniques [10]. Since Dentoalveolar abscess are induced by microorganisms that are part of the normal oral microflora, it is therefore extremely important to disinfect the mucosal surface overlying the abscess, thus, a variety of sampling techniques have been used in past studies, previous studies have used iodine solution for surface disinfection. [11,12] few used 70% ethyl alcohol solution [13,14].
In our study for each patient, the oral mucosa overlying the abscess was scrubbed with tincture of iodine; a sterile 18- gauge needle fitted to a 3-ml disposable syringe was passed through the alveolar mucosa into the abscess, from which the contents are withdrawn, the needle was sealed immediately by cork. In our study after the aspiration the needle was sealed immediately using wooden cork, the specimens were transported in the same syringe to microbiology laboratory and processed within 30 mins, this method was employed in the previous studies [15,16]. Shah A et al. used culture media containing chocolate agar or Mac Conkey agar medium. Plates were incubated at 37°C for 12– 24 h in an aerobic atmosphere [17]. In a study by Kityamuwesi R et al. specimen was immediately inoculated in a transport medium, Soybean casein digest broth - BD BACTEC Plus + Anaerobic/F Medium. [14] Habib A et al inoculated swab immediately into a tube of thioglycollate broth, the specimens were incubated for 24 hours at 37°C. Then subcultured onto 2 solid agar plates, one blood agar plate for aerobic incubation for 24 hours and one brain heart infusion agar for anaerobic incubation for 48-72 hours [18]. The use of an anaerobic chamber is currently considered the best technique available for recovery of stringent anaerobes since specimens and cultures can be protected from oxygen at every stage of the procedure.
Our study has revealed 90% positive cultures of anaerobic microorganisms (54/60 cases), total of 92 isolates were recovered, accounting for 1.53 isolates per specimen in non-Diabetic, whereas 95% positive cultures of anaerobic microorganisms (57/60 cases), total of 116 isolates were recovered, accounting for 1.95 isolates per specimen in Diabetic patients, our study shows significantly higher isolates per specimen in Diabetic patients. A study by Al- Farhan S.R et al. revealed that bacterial flora frequency is changing, the Gram-negative bacterial isolates are common and Grampositive isolates are significantly less (p< 0.05) in Diabetic patients [8].
In our study, commonest isolation s were Peptostreptococcus, Peptococccus, Fusobacterium, Prevotella and Bacteroides in non-Diabetics incidence was 35.86%, 31.52%, 17.4%, 8.7%, and 6.52% respectively and in Diabetics incidence was 26.72%, 22.41%, 25.00%, 16.37% and 9.48% respectively, incidence of isolates were comparable between the groups. In few studies Fusobacterium is frequently reported in infections of the head and neck with reports indicating that Fusobacterium species can be detected in up to 52% of specimens [19]. It is worth visualizing, that isolated microorganisms are not same in each case but differ from one individual to other, i.e., Bacteroides, Fusobacterium, Peptostreptococcus, Peptococcus and Eubacterium and these bacteria have been found out to be the main causative agent of endodontic infection [20]. A study by Habib A et al, showed 87 patients with (58.0%) had anaerobic bacterial infection. 71 patients (47.3%) had single bacterial isolate and 16 patients (10.7%) had multiple bacterial isolates. The most common isolated organism was Prevotella spp. (63 patients 42%) [18].
Among the entire anaerobically cultured bacteria, Metronidazole was the most sensitive drug (100%) in both the groups followed by, Amoxicillin/clavulanic acid (90% and 95%), Clindamycin (85% and 83%) whereas Ciprofloxacin is least sensitive (p<0.0001) in Diabetic patients, The least effective drug was amoxicillin (100%) in both the groups. Kityamuwesi R et al concluded that all Viridans Streptococci isolates were resistant to penicillin G, ampicillin and tetracycline, but retained susceptibility to vancomycin, all Staphylococcus aureus strains were resistant to cotrimoxazole and, but susceptible to vancomycin, and amoxicillin/ clavulanate. All the gram-negative isolates were susceptible to amikacin and imipenem, but had poor susceptibility rates to ceftazidime, cotrimoxazole and ampicillin [14]. In a study by Rugarabamu et al. majority of these organisms were susceptible to β-Lactam and β-lactamase inhibitor antibiotics such as Penicillin, Clindamycin, Metronidazole, Cefalosporin and Carbapenem [21]. Habib A et al, isolated Peptostreptococcusspp (26.7%) in 40 instances in which Metronidazole was the most sensitive drug (100%) followed by Amoxicillin/ clavulanic acid (87.2%), Clindamycin (84.6%), Cefotaxime and Ciprofloxacin (71.8%) each. The most resistant drugs were amoxicillin (100%). Prevotellaspp (42%) was isolated in 63 instances in which Metronidazole was the most sensitive drug (100%) followed by Amoxicillin/clavulanic acid (90.5%), Clindamycin and Ciprofloxacin (88.9%) each [18].
The appearance of penicillin in the market and clinical use has saved many lives throughout the Planet, transformed medical science and its success in treating infectious disease [22]. Poor use of antimicrobials promotes the emergence of antibiotic-resistant microbial strains, [23] increases the possibility of antibiotic-associated adverse reactions. Multiple studies reported that dental surgeons frequently prescribed inappropriate antibiotics which ultimately promote antimicrobial resistance [24]. The results of antibiotic susceptibility revealed the need to further studies to investigate variation in antimicrobial resistance.
Conclusion
Thus, the present study has highlighted that anaerobes were significantly more associated with cases of Diabetes mellitus, the most common anaerobic bacteria are Peptostreptococcus species, Prevotela species and Fusobacterium species. The choice of empiric antimicrobial agents in diabetic patients should take into account the preponderance of both Gram positive and Gram Negative anaerobes. . However, further research using a broad range molecular microbiological method for identifying a greater number of pathogens may be necessary to demonstrate a significant association with Diabetes mellitus.
Financial Support
Nil.
Conflict of Interest
There are no conflicts of interest.
Acknowledgement
The authors are incredibly grateful for the expert assistance with review of this article provided by Dr. Pratibha malini. J. Professor, Department of Microbiology, KIMS Hospital and Research centre, Bengaluru, Karnataka.
References
- Rusinovci S, Sejdini M, Salihu S, et al. Bacteria that cause dentoalveolar abscesses after failed endodontic treatments: A pilot study. J Int Dent Med Res 2018; 11:823-829.
- Walsh LJ. Novel Approaches to detect and treat biofilms within the root canals of teeth: A review. Antibiotics 2020; 9:129.
- Topazian RG, Goldberg MH, Hupp JR. Oral and maxillofacial infections. 4th edition. Philadelphia, WB. Saunders 2002.
- Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care 2003; 26:510-513.
- Haug RH. The changing microbiology of maxillofacial infections. Oral Maxillofac Surg Clin N Am 2003; 15:1-15.
- Siqueira JF, Rocas IN. PCR methodology as a valuable tool for identification of endodontic pathogens. J Dent 2003; 31:333-339.
- Beighton D, Lynch E, Comparison of selected microflora of plaque and underlying carious dentine associated with primary root caries lesions. Caries Res 1995; 29:154-158.
- Al-Farhan SR, AL-Abdullah AA, Al-Moussawi AA. Isolation and diagnosis of anaerobic bacteria of periodontitis by molecular methods in diabetic and non-diabetic patients in basra province/Iraq”. Sci J Med Res 2019; 3:53-63.
- Blome B, Braun A, Sobarzo V, et al. Molecular identification and quantification of bacteria from endodontic infections using real-time polymerase chain reaction. Oral Microbiol Immunol 2008; 23:384-390.
- Turner JE, Moore DW, Shaw MT. Prevalence and antibiotic susceptibility of organisms isolated from acute soft tissue abscesses secondary to dental caries. Oral Surg 1975; 39:848855.
- Reddy PA, Jyothi PN, Rajendran M. Evaluation of microbial flora and antibiotic sensitivities in orofacial space infections of odontogenic origin. Int J Health Sci Res 2019; 9:105-110.
- Heim N, Faron A, Wiedemeyer V, et al. Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. Differences in inpatient and outpatient management. J Cranio Maxillofac Surg 2017; 45:1731-1735.
- Vishnoi N, Singh S, Bishnoi RS, et al. Antibiotic evaluation of odontogenic microbiological spectrum of orofacial infection. J Drug Delivery Therapeutics 2018; 8:179-182.
- Kityamuwesi R, Muwaz L, Kasangaki A, et al. Characteristics of pyogenic odontogenic infection in patients attending Mulago Hospital, Uganda: A cross-sectional study. BMC Microbiol 2015; 15:1.
- Bahl R, Sandhu S, Singh K, et al. Odontogenic infections: Microbiology and management. Contemporary Clin Dent 2014; 5:307.
- Brito TP, Hazboun IM, Fernandes FL, et al. Deep neck abscesses: Study of 101 cases. Br J Otorhinolaryngol 2017; 83:341-348.
- Shah A, Ramola V, Nautiyal V. Aerobic microbiology and culture sensitivity of head and neck space infection of odontogenic origin. Natl J Maxillofac Surg 2016; 7:56-61.
- Habib A, Elbokle N, Hakam M. Maxillofacial infections of odontogenic origin: Odontopathogens and antibiotic sensitivity: A demographic cross-sectional study in Elsharqia Governorate. Egyptian J Oral Maxillofac Surg 2019; 10:20-26.
- Bronzato JD, Bomfim RA, Edwards DH, et al. Detection of fusobacterium in oral and head and neck cancer samples: A systematic review and meta-analysis. Archives Oral Biol 2020; 112:104669.
- de Lima Guimarães NL, Otoch HM, de Andrade LC, et al. Microbiological evaluation of infected root canals and their correlation with pain. Rev Sul-Bras de Odontol 2012; 9:31
- Rugarabamu SE, Simon EM, Matee MI. Use of clinical clue to diagnose anaerobic oral and maxillofacial infections among patients at Muhimbili national hospital, Dar-es-Salaam, Tanzania. African J Microbiol Res 2017; 11:422-425.
- Adedeji WA. The treasure called antibiotics. Ann Ib Postgrad Med 2016; 14:56–57.
- Bryce A, Hay AD, Lane IF, et al. Global prevalence of antibiotic resistance in pediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: Systematic review and meta-analysis. BMJ 2016; 352:i939.
- Teoh L, Stewart K, Marino RJ, et al. Current prescribing trends of antibiotics by dentists in Australia from 2013 to 2016. Aust Dent J 2018; 63: 329–337.
Author Info
Department of Dentistry, Bowring Lady Curzon Medical College and Research Institute, Bangalore, Karnataka, IndiaCitation: Ranjith Raj VPRB, Murugan Thamaraiselvan, Incidence and Antibiotic Sensitivity of Anaerobic Bacteria in Dentoalveolar Abscess of Diabetic and Non-Diabetic Patients: A Cross Sectional Comparative Study, J Res Med Dent Sci, 2020, 8 (7): 428-434.
Received: 22-Sep-2020 Accepted: 16-Nov-2020 Published: 23-Nov-2020