Review Article - (2023) Volume 11, Issue 1
Investigation the Activity of Iraqi Agave attenuata on In Vitro Growth of Cutaneous Leishmania Promastigotes
Sherine Majeed Shah* and Thukaa Z Abdul Jalil
*Correspondence: Sherine Majeed Shah, Department of Pharmacognosy, College of Pharmacy, University of Baghdad, Baghdad, Iraq, Email:
Abstract
In this research we concentrate on the study of the activity of Iraqi Agave attenuata root and leaves as antileishmania (cutaneous leishmania). Focusing on the phenolic content of the plant that detected in high concentration in ethyl acetate fraction obtained by extraction method using soxhlet apparatus. Also depending on the saponin content that Agave species rich with these constituents, in addition because of the previously studied effect of saponin as anti-parasite encourage us to extract steroidal saponin by a particular way. The result was the phenolic constituents in root have a great antileishmanial activity. As well as steroidal saponin root and leaves showed a considerable effect that is identical and also better than the drug used (pentostam) as a control. This study is the first to describe the antileishmanial activity especially on cutaneous leishmania and the result showed that the concentration that gave us perfect effect in ethyl acetate root fraction was at 1 mg/ml with IC50 (0.768 mg/ml), for steroidal saponin leaves and root fractions the maximum effect were at 1 mg/ml and 0.125 mg/ml respectively with IC50 (0.48 mg/ml) for leaves and (0.6 mg/ml) for root.
Keywords
Agave attenuata, Phenolic compounds, Saponins, Cutaneous leishmania, Pentostam
Introduction
The high toxicity of the currently available chemotherapeutic treatments makes it difficult to control leishmaniasis. The continued hunt for more powerful leishmanicidal chemicals has forced herbal medications into the spotlight as a safe and effective alternative to conventional therapies, which have a number of downsides. Leishmaniasis is a group of diseases caused by protozoan parasites belonging to the Leishmania genus. They are found in 98 countries and have a wide range of clinical manifestations, from disfiguring skin lesions known as Cutaneous Leishmaniasis (CL) to the fatal visceral variant of the illness known as Visceral Leishmaniasis (VL) or kala-azar [1].
Human to human transmission (anthroponotic transmission) or animal to human transmission (zoonotic transmission) are also possible ways for the disease to spread. Parasites (infective metacyclic promastigote forms) are transmitted during the hematophagy of the insect vector in both circumstances [2].
Infectious parasites are phagocytized by macrophages after being implanted in the dermis epidermis junction of vertebrate hosts and then begin the differentiation process to amastigotes, the evolutive form responsible for disease development and progression [3]. Because macrophages play such an important part in the infection as host cells, finding medicines that can trigger an effective antileishmanial response from these cells is critical.
The medications employed have varying efficacies and significant toxicity (cardiotoxicity, hepatotoxicity and nephrotoxicity, for example), which can cause a variety of side effects. The emergence of parasite drug resistance adds to the difficulty of controlling leishmaniasis [4].
Nowadays the prospection of plant species as an approach for discovering more effective compounds against leishmaniasis has been the attention of various research laboratories across the world.
The monocotyledonous family Agavaceae includes the genus Agave. Around 166 species of Agave exist [5,6]. The genus Agave originated in Mexico. This country has the most Agave species, with 125 species growing primarily in dry and semiarid zones. This genus' members produce a variety of secondary metabolites, which are employed in traditional medicine as anticancer, anti-inflammatory and anti-parasitic agents, among other things [7]. Saponins are abundant in Agave species and they are the most investigated secondary metabolites in that genus [8-13].
However, Agave species produce a large number of phenolic chemicals, which are both diverse and abundant. Salm-Dick Agave attenuate, this is one of Agave species that has recently been studied for its phenol and flavonoid content as well as its biological activity [14].
Because Agave species are rich in phenolic compounds and steroidal saponins and there are a lot of studies about the effect of saponins as anti-parasite and especially steroidal saponins in addition to other evidence that phenolic compound have a good effect as antileishmaniasis that encourage us to discover this important activity in Agave attenuate leaves and root that never studied before [15-17].
Materials and Methods
Plant material
From local botanical garden in Baghdad fresh leaves and root were collected. Then these fresh leaves and root were identified by department of biology/biology university Baghdad college and authentication done by Dr. Zainab Abed Aoun PhD in plant identification.
Preparation of plant extract
500 grams of fresh leaves and 250 gm of fresh root of Agave attenuata were washed to remove other external materials, dried in shade place with natural air until completely dried, then powdered by using electric blender and weighed for extraction method
Extraction method for different phyto constituents by using soxhlet apparatus (for phenolic and flavonoids phyto constituents)
80 grams of powdered Agave attenuate leaves and root were packed in a thimble and extracted using 750 milliliters of n-hexane for four hours for the leaves and root, respectively. The extract was then dried until the crude extract weighed 1.9 gm (leaves) and 0.4 gm (root) and the plant was then allowed to dry before continuing extraction with another solvent. Ethyl acetate was the other solvent used. Place the dried plant in a thimble and extract with 750 ml of ethyl acetate for 7 hours, or until the extract turns pale in color. The crude extracts were also dried and weighed, yielding 0.8 gm (leaves) and 0.9 gm (root). The plant was also allowed to dry to carry on with the extraction process. The final solvent was methanol and the dried plant was packed in a thimble with 800 ml of methanol and the extraction began and lasted for 20 hours until the color turned pale. After drying, the crude extract weighed 10.9 gm (leaves) and 2.7 gm (root) [18].
Extraction method for steroidal saponin
30 gram dried powdered leaves and root suspended in 400 ml distilled water, refluxed for 3 hours to separate the polysaccharides, the aqueous extract was filtered and dried before being re suspended in an ethanol: water (80:20) solution and left at 25°C for 18 hours. Filtration was used to separate the polysaccharides and the hydro alcoholic extract was dried. By separator funnel, the concentrated hydro alcoholic was partitioned with ethyl acetate (1:2, v/v, 2 times) and the ethyl acetate layer was dried.
The ethyl acetate extract was then hydrolyzed in an 80 percent hydro ethanolic solution under reflux for 3 hours (30 ml containing 5 ml of concentrated HCL).
The reaction mixture was neutralized with 10% NaOH before being extracted twice with ethyl acetate using a separating funnel (150 ml first time and then 100 ml).
The ethyl acetate layer was evaporated till dry, yielding 0.4 gram leaves and 0.6 gm root, which were subsequently identified [19,20].
Antileishmanial activity
Preparation of inoculum: Cutaneous leishmania cells obtained from many confirmed cases collected from hospitals in Baghdad and brought to Al-Nahrain university's college of science's center for biological technology research. The antileishmanial activity of plant extract was tested by first propagating the organism in log phase in RPMI media (Roswell Park Institute Park Memorial) enriched with 12% serum from calf fetal, at 25°C for 3 days until an average of 105 parasites/ml in haemocytomer was reached [21,22].
Preparation of plant extract concentrations
Anti-parasitic activity was examined in four fractions and compared to a commonly used antilieshmania medicine. Because of the availability of high quantities of phenolic phyto constituents, the ethyl acetate fraction for root and leaves obtained by hot method extraction (soxhlet apparatus) and labeled as (A) for Ethyl Acetate Leaves (EAL) and as (B) Ethyl Acetate Root (EAR) were chosen. The steroidal saponin fractions obtained from the fore mentioned method of extraction were labeled as (C) Steroidal Saponin Leaves (SSL) and as (D) Steroidal Saponin Root (SSR). Each fraction was weighed at 1 mg and kept for five repeated dilutions. Dimethyl Sulfoxide (DMSO) in 100% v/v was added as a solubilizing agent to each of the four plant extracts in an amount that did not exceed 20 μL, followed by distilled water until the required concentration (1 mg/ml) was reached, which was then used to prepare successive dilutions. 1000 μg/ ml, 500 μg/ml, 250 μg/ml, 125 μg/ml and 62.5 μg/ml were the concentrations that were obtained.
Preparation of positive control
The positive control was GlaxoSmithKline/UK pentavalent antimonial (sodium stibogluconate injection 100 mg/ml), which is a typical antileishmania medication. The sodium stibogluconate (100 mg/ml) was made by diluting 1 ml of medication in 10 ml distilled water to a concentration of 10 mg/ml (10000 μg/ml). In each well, six microliters of RPMI medium and one microliter of inoculum were inoculated separately. To ensure reproducibility, the experiments were repeated three times [23].
Evaluation of the sample fractions against cutaneous leishmaniasis
To assess antileishmanial activity, flat bottom plate that contained 96 wells, (Figure 1) were 200 μL (x3) were taken from each concentration that were previously prepared, a total of 60 wells were occupied, another 12 wells with culture only as negative control, twelve wells with the positive control of sodium stibogluconate and the last twelve wells were a blank which only contained 100 μl of culture medium without any plant extract, drug or parasite. The plate was incubated at 25°C ± 1°C for 24 hours after which a 10 μL. MTT dye (3-(4,5-dimethyl thiazo-2-yl)-2,5-diphenyltetrazolium bromide) was added in each well then incubated again for further 4 hours at 25°C ± 1°C to assess metabolic activity, followed by addition of DMSO to each well as a solubilizing agent that will target the MTT purple dye inside the living matter release and making scanning process possible [23].
Figure 1: 96 wells plate that is ready for scan with ELISA.
Scanning
The ELISA (Enzyme Linked Immuno sorbent Assay) spectrophotometer instrument detects the optical density in each well at a wavelength of 490 nm and the higher the number of living matter, the more purple the color presented and the higher the ELISA reading absorbance.
Statistical analysis
The significance of the data was verified using a mean comparison after calculating the inhibition rate percentage of each mean for the three fraction concentration gradients used. Using IBM software's one way ANOVA test, the Least statistically Significant Differences (LSD) and p value to determine whether the inhibition rate is significantly different from that of sodium stibogluconate.
Calculating of IC50
While it's essential to know how to distinguish between different types of inhibitors, much of the drug development process is focused on establishing whether one inhibitor is more effective than another. This requires determining the potency of an inhibitor. The IC50 is the most commonly used to quantify an inhibitor's effect. The IC50 value is the inhibitor concentration required to lower the rate of an enzymatic process by 50% [24].
The IC50 of each analytical sample was calculated using the following procedure: at each of the five locations, inhibition ratios (y) were plotted against sample concentrations (x) and the associated regression line (y=ax+b) was generated. It was not necessary for the regression line to pass through the origin. Because the inhibition curve was not precisely straight but slightly curved, we can determine the IC50 value using interpolation by connecting the two points surrounding the 50% inhibition point with a straight line. After selecting two points surrounding a 50% inhibition ratio, a regression line (y=ax+b) was created. The regression line did not have to pass through the origin; instead, x (sample concentration) was determined by substituting 50 for y in the regression equation y=ax+b [25].
Results and Discussion
Extraction and fractionation
The composition of the plant material, the solvent used, the pH of the solvent, the temperature and the solvent to sample ratio all influence the extraction method chosen. It also relies on how the final products will be used. The type of plant, the section of the plant to be extracted, the nature of the bioactive components and the availability of solvent all influence the solvent choice. In general, polar solvents like water, methanol and ethanol are used in polar compound extraction, while nonpolar solvents like hexane and dichloromethane are used in nonpolar compound extraction [26]. To extract all phytochemicals that found in the Iraqi Agave attenuata we used soxhlet apparatus with increasing polarity solvents.
Steroidal sapogenins, commonly known as C-27 steroidal saponins, are mainly found as glycosides. The hydro alcoholic extract's ethyl acetate partition was found to be selective for saponins as aglycone. Sapogenins can be extracted using non-polar solvents or supercritical fluids after chemical, enzymatic, or hydrothermal hydrolysis of crude saponins and/or saponin rich extracts. Mineral acids (such as HCL and H2SO4) are typically employed in the traditional approach. The liquid-liquid extraction with ethyl acetate demonstrated good selectivity for saponins. The limited solubility of saponins as an aglycone in aqueous solution could explain the ethyl acetate extract's high selectivity for obtaining saponins.
Antileishmanial activity test
The percent of organisms killed by each concentration of each tested fraction was calculated using the Optical Density (OD) data collected from ELISA, using the equation:
% Inhibition Rate=(OD Control-OD Test/OD control) × 100

The % inhibition rate was calculated for each concentration of each fraction and compared to the computations of the positive and negative controls, as well as ANOVA analysis to determine the concentration that there is no significant mean difference between this and the positive control (the null hypothesis is to be achieved) at (p>0.05).
Fraction 1 (EAL): The calculated inhibition rate and mean for ethyl acetate leaves' five concentrations revealed that there is low activity as antileishmaiasis comparing with the positive control inhibition rate that is 56.25% (Table 1).
| Sample name | Concentrations | % IR Means ± SD |
|---|---|---|
| A1 | 1 mg/ml | 9.745471 ± 0.279610443 |
| A2 | 0.5 mg/ml | 24.84904 ± 0.085359371 |
| A3 | 0.25 mg/ml | 37.38439 ± 0.030214051 |
| A4 | 0.125 mg/ml | 36.4366 ± 0.123776681 |
| A5 | 0.625 mg/ml | 38.88252 ± 0.090691173 |
Table 1: Mean of % IR for each concentration gradient for fraction A.
Fraction B (EAR): The % IR and mean comparison calculations revealed that concentration at 1 mg/ml (B1) has killing effect to the cells more than that of positive control (% IR 56.24856684) and % IR for B1 is 71.32156233% (Table 2).
| Sample name | Concentrations | % IR Means ± SD |
|---|---|---|
| B1 | 1 mg/ml | 71.32156233 ± 0.162735641 |
| B2 | 0.5 mg/ml | 17.23610793 ± 0.245993677 |
| B3 | 0.25 mg/ml | 21.94450814 ± 0.562203403 |
| B4 | 0.125 mg/ml | 2.835741038 ± 0.038212854 |
| B5 | 0.625 mg/ml | 7.360697088 ± 0.043135446 |
Table 2: Mean of % IR for each concentration gradient for fraction B.
Fraction C (SSL): The % IR and mean comparison calculations revealed that concentration at C1 (1 mg/ml) has the inhibition rate greater than that of positive control, the % IR of +ve is 56.24856684% and % IR of C1 is 73.40059619% also at concentration C3 (0.25 mg/ ml) and C4 (0.125 mg/ml) show activity close to that of positive control (Table 3).
| Sample name | Concentrations | % IR Means ± SD |
|---|---|---|
| C1 | 1 mg/ml | 73.40059619 ± 0.072350996 |
| C2 | 0.5 mg/ml | 28.05931361 ± 0.117252813 |
| C3 | 0.25 mg/ml | 55.78995643 ± 0.183798803 |
| C4 | 0.125 mg/ml | 52.7325537 ± 0.283033959 |
| C5 | 0.625 mg/ml | 24.3598563 ± 0.430743801 |
Table 3: Mean of % IR for each concentration gradient for fraction C.
Fraction D (SSR): The % IR and mean comparison calculations revealed that the concentrations at D3 (0.25 mg/ml), D4 (O.125 mg/ml) and D5 (0.625 mg/ml) showed greater activity than positive control rate. And at concentration D2 (0.5 mg/ml) displayed very close effect to positive control (Table 4).
| Sample name | Concentrations | % IR Means ± SD |
|---|---|---|
| D1 | 1 mg/ml | 34.4187113 ± 0.173305126 |
| D2 | 0.5 mg/ml | 56.61545517 ± 0.203592731 |
| D3 | 0.25 mg/ml | 65.81823741 ± 0.024931016 |
| D4 | 0.125 mg/ml | 66.24627379 ± 0.116610463 |
| D5 | 0.625 mg/ml | 61.99648399 ± 0.296154614 |
Table 4: Mean of % IR for each concentration gradient for fraction D.
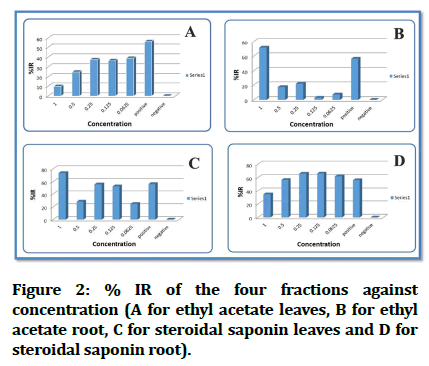
In Figure 2 we can see the inhibition rate % IR against concentrations and comparing the result with the positive control used (Figure 2).
Figure 2: % IR of the four fractions against concentration (A for ethyl acetate leaves, B for ethyl acetate root, C for steroidal saponin leaves and D for steroidal saponin root).
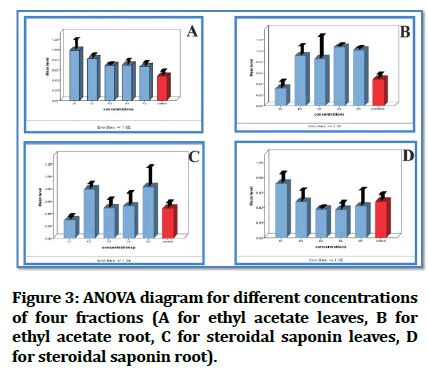
ANOVA analysis revealed equivalent and non-significant differences between fractions and positive control, which is the goal in this study to achieve a similar effect to that of the control (Figure 3).
Figure 3: ANOVA diagram for different concentrations of four fractions (A for ethyl acetate leaves, B for ethyl acetate root, C for steroidal saponin leaves, D for steroidal saponin root).
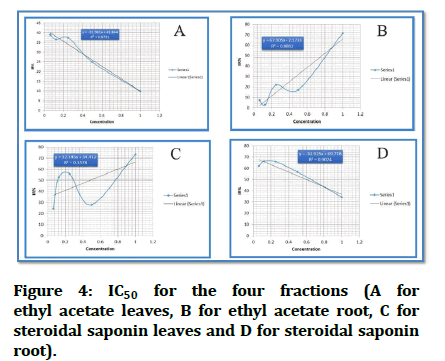
IC50: The IC50 of each analytical sample was calculated using the following procedure: At each of the five locations, inhibition ratios (y) were plotted against sample concentrations (x), and a regression line (y=ax+b) was generated.
Unfortunately, the computed IC50 for fraction ethyl acetate leaves EAL did not exist since the fraction lacks the ability to kill 50% of infected cells.
According to the straight line equation, the computed IC50 for second fraction ethyl acetate root EAR is 0.768 mg/ml.
The IC50 for steroidal saponin leaves SSL is 0.48 mg/ml calculated from straight line equation.
IC50 for the last fraction, steroidal saponin root SSR, is 0.6 mg/ml (Figures 3 and 4).
Figure 4: IC50 for the four fractions (A for ethyl acetate leaves, B for ethyl acetate root, C for steroidal saponin leaves and D for steroidal saponin root).
For the first time, antileishmanial activity was estimated for the plant Agave attenuata leaves and root for fractions rich in phenolic phyto constituents (ethyl acetate for leaves and root), as well as because Agave species are full of steroidal saponin and the activity of saponin as an anti-parasitic caught our attention, so we extracted steroidal saponin in a special method for leaves and root and tested for antileishmania effect. These fractions have antileishmanial activity, and in certain amounts (Table 5).
| Fraction name | Fraction content | Concentration used | % IR |
|---|---|---|---|
| A | EAL | 0.625 mg/ml | 38.88% |
| B | EAR | 1 mg/ml | 71.32% |
| C | SSL | 1 mg/ml | 73.40% |
| D | SSR | 0.125 mg/ml | 66.25% |
Table 5: Concentration of each fraction that showed best antileishmanial activity.
As previously stated, the antileishmanial activity of steroidal saponin in leaves and root is very good and phenolic phytoconstituents in root have a significant effect, making them a suitable candidate for further research as antileishmanial substances.
Conclusion
In this study, the Iraqi Agave attenuata leaves and root were discovered as a natural medicinal plant that contain many phyto constituents that proved their ability to act as antileishmania identical to that of pentostam treatment.
Four fractions used for this experiment depending on the high concentration of phenolic constituents that found in ethyl actate fraction for leaves and root. The other two fractions, we focused on steroidal saponin content of Agave attenuata that the saponin has anti-parasitic activity. The result was the phenolic content in ethyl acetate fraction for root has a considerable activity at 1 mg/ml, and has IC50 at 0.768 mg/ml. Both steroidal saponin fractions also showed a significant effect, for leaves the maximum activity was at 1 mg/ml and the IC50 appear at 0.48 mg/ml and about the steroidal saponin fraction for root, the highest activity was at 0.125 mg/ml and IC50 was at 0.6 mg/ml.
References
- Hartley MA, Ronet C, Zangger H, et al. Leishmania RNA virus: When the host pays the toll. Front Cell Infect Microbiol 2012; 2:99. [Crossref][Googlescholar][Indexed]
- Chakravarty J, Sundar S. Drug resistance in leishmaniasis. J Glob Infect Dis 2010; 2:167. [Crossref][Googlescholar][Indexed]
- de Pablos LM, Ferreira TR, Walrad PB. Developmental differentiation in leishmania lifecycle progression: Post-transcriptional control conducts the orchestra. Curr Opin Microbiol 2016; 34:82–89. [Crossref][Googlescholar][Indexed]
- Sundar S, Chakravarty J. An update on pharmacotherapy for leishmaniasis. Expert Opin Pharmacother 2015; 16:237-252. [Crossref][Googlescholar][Indexed]
- Good-Avila S V, Souza V, Gaut BS, et al. Timing and rate of speciation in Agave (Agavaceae). Proc Natl Acad Sci USA, 2006; 103:9124–9129. [Crossref][Googlescholar][Indexed]
- Rocha M, Good Avila S, Molina Freaner F, et al. Pollination biology and adaptive radiation of Agavaceae, with special emphasis on the genus Agave. Aliso 2006; 22:329–344. [Googlescholar][Indexed]
- Santos Zea L, Leal Diaz A, Cortes Ceballos E, et al. Agave (Agave spp.) and its traditional products as a source of bioactive compounds. Curr Bioact Compd 2012; 8:218–231. [Crossref]
- Bodeiko VA, Kintya PK. The structure of Agave Saponins C’ and D from the leaves of Agave americana. Steroid Sapon 1975; 11:755–777.
- Debnath M, Pandey M, Sharma R, et al. Biotechnological intervention of Agave sisalana: A unique fiber yielding plant with medicinal property. J Med Plants Res 2010; 4:177–187. [Googlescholar][Indexed]
- Pant G, Sati OP, Miyahara K, et al. Search for molluscicidal agents: Saponins from Agave cantala leaves. Int J Crude Drug Res 1987; 25:35–38. [Crossref][Googlescholar][Indexed]
- Tinto WF, Simmons Boyce JL, McLean S, et al. Constituents of Agave americana and Agave barbadensis. Fitoterapia 2005; 76:594–597. [Crossref][Googlescholar][Indexed]
- Yokosuka A, Jitsuno M, Yui S, et al. Steroidal glycosides from Agave utahensis and their cytotoxic activity. J Nat Prod 2009; 72:1399–1404. [Crossref][Googlescholar][Indexed]
- Eskander J, Lavaud C, Harakat D. Steroidal saponins from the leaves of Agave macroacantha. Fitoterapia 2010; 81:371–374. [Crossref][Googlescholar][Indexed]
- Rizwan K, Zubair M, Rasool N, et al. Phytochemical and biological studies of Agave attenuata. Int J Mol Sci 2012; 13:6440–6451. [Crossref][Googlescholar][Indexed]
- Sparg SG, Light ME, Van Staden J. Biological activities and distribution of plant saponins. J Ethnopharmacol 2004; 94:219–243. [Crossref][Googlescholar][Indexed]
- Dutta A, Ghoshal A, Mandal D, et al. Racemoside A, an anti-leishmanial, water soluble, natural steroidal saponin, induces programmed cell death in Leishmania donovani. J Med Microbiol 2007; 56:1196–1204. [Crossref][Googlescholar][Indexed]
- Antwi CA, Amisigo CM, Adjimani JP, et al. In vitro activity and mode of action of phenolic compounds on Leishmania donovani. PLoS Negl Trop Dis 2019; 13:1–22. [Crossref][Googlescholar][Indexed]
- Galanakis CM, Goulas V, Tsakona S, et al. A knowledge base for the recovery of natural phenols with different solvents. Int J Food Prop 2013; 16:382–396. [Crossref][Googlescholar][Indexed]
- Santos JDG, Branco A. GC-MS characterization of sapogenins from sisal waste and a method to isolate pure hecogenin. Bio Resources 2014; 9:1325–1333. [Crossref][Googlescholar][Indexed]
- Abrahem RM, Awad ZJ. Phytochemical study of steroidal sapogenin tigogenin present in the leaves of Agave americana cultivated in Iraq. Iraqi J Pharm Sci 2015; 24:41-47. [Crossref][Googlescholar][Indexed]
- Bansal D, Sehgal R, Chawla Y, et al. In vitro activity of anti-amoebic drugs against clinical isolates of Entamoeba histolytica and Entamoeba dispar. Ann Clin Microbiol Antimicrob 2004; 3:1–5. [Crossref][Googlescholar][Indexed]
- Sereno D, Lemesre JL. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother 1997; 41:972–976. [Crossref][Googlescholar][Indexed]
- Al-Ogaili N. Synergistic effect of Lawsonia inermis and Peganum harmala aqueous extracts on in vitro growth of Leishmania tropica promastigotes comparison to sodium stibogluconate. Al-Qadisiyah Med J 2016; 12:76-83.[Googlescholar][Indexed]
- Martinez Morales F, Alonso Castro AJ, Zapata Morales JR, et al. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem Pap 2020; 74:3325–3334. [Crossref][Googlescholar][Indexed]
- Abubakar AR, Haque M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci 2020; 12:1–10. [Crossref][Googlescholar][Indexed]
- Gontijo VS, Espuri PF, Alves RB, et al. Leishmanicidal, antiproteolytic and mutagenic evaluation of alkyl triazoles and alkyl phosphocholines. Eur J Med Chem 2015; 101:24–33. [Crossref][Googlescholar][Indexed]
Author Info
Sherine Majeed Shah* and Thukaa Z Abdul Jalil
Department of Pharmacognosy, College of Pharmacy, University of Baghdad, Baghdad, IraqCitation: Sherine Majeed Shah, Thukaa Z Abdul Jalil, Investigation the Activity of Iraqi Agave attenuata on In Vitro Growth of Cutaneous Leishmania Promastigotes, J Res Med Dent Sci, 2023, 11 (01): 114-120.
Received: 01-Nov-2022, Manuscript No. JRMDS-23-66424; , Pre QC No. JRMDS-23-66424 (PQ); Editor assigned: 04-Nov-2022, Pre QC No. JRMDS-23-66424 (PQ); Reviewed: 18-Nov-2022, QC No. JRMDS-23-66424; Revised: 28-Dec-2022, Manuscript No. JRMDS-23-66424 (R); Published: 05-Jan-2023