Research - (2022) Volume 10, Issue 3
Involvement of IL-23 and IL-12 Levels in the Development of PeriâImplantitis
Ehab Qasim Talib* and Ghada Ibrahim Taha
*Correspondence: Ehab Qasim Talib, Department of Basic Sciences, College of Dentistry, University of Baghdad, Baghdad, Iraq, Email:
Abstract
Dental implants: a surgical component that interfaces to the jaw or skull bone to support a dental prosthesis such as a crown, bridge, denture, or facial prosthesis. The aim of this study was to detect the role and related inflammatory cytokines IL-23 and IL-12 in the initiation and progress and evaluate their implication in peri-implant inflammation and their influence upon osseointegration. Material and Methods: 80 subjects (15 peri-implantitits patients and 35 successful implants and 30 healthy controls); the mean age was 43.91 ± 11.33 years, (49 male and 31 female), were attending the department of Oral and Maxillofacial Surgery in the Dental College Teaching Hospital/ Baghdad University, and Shahid Ghazi Hariri Hospital/ Medical City Baghdad, Iraq. Enzyme-linked immunosorbent assay (ELISA) kits were used for the analysis and detection of IL-12 and IL-23. Results: The elevated PISF levels of selected inflammatory cytokines is a result of their activation in the inflammatory process of the peri-implantitis, however, this activation may contribute to peri-implant supportive tissue destruction and bone loss. Conclusion: These results suggest that IL-23 may play an important role in inflammation-mediated bone loss. However, IL-12 exhibits potential as a target for the treatment of pathological bone diseases.
Keywords
Dental implants, Peri–implantitis, Per-implant sulcular fluid, IL-23/ IL-12
Introduction
Dental implants are one of the most wellestablished and reliable treatments in contemporary dentistry. This surgical component connects with the jaw or skull bone to support a dental prosthesis such as a crown, bridge, denture, facial prosthesis, or function as an orthodontic anchor. The most common and proven implant materials are titanium and titanium alloys, which have demonstrated excellent tissue integration. For materials like titanium to develop a close relationship with the bones, osseointegration is required [1]. A load-bearing implant and organized live bone form a direct structural and functional link [2]. Insufficient osseointegration by the host tissue may contribute to implant failure [3]. Implant failures are classified as early or late failures, or as infectious or noninfectious. Also, Early failures occur due to surgical damage, premature loading of the implant, or bacterial infection, whereas late failures occur due to the implant's inability to sustain osseointegration [3-5]. However, removing the tooth and implanting it causes inflammation in the jawbone and body, leading to uneventful recovery and implant stability [6]. An inflammatory response is used to contain pathogenic bacteria, draw cells and their products to injured regions, and start the healing process. It includes various pathological processes and cell types. Despite the unpleasant symptoms, these responses help to isolate a disease and prevent it from spreading [7]. Pathogenic bacteria may colonise dental implants, causing inflammation of the supporting tissues [8]. Contrary to popular belief, bacterial biofilm production does not cause tissue harm in peri implantitis. Multiple studies have indicated that titanium implants may create chronic inflammation in the surrounding tissue, causing local and systemic health issues [9]. On the basis of immunological vulnerability to Th I and macrophage reactivity which may eventually lead to titanium implant failure, periimplant illness, peri-implant mucositis, and peri-implantitis have been postulated [6]. Periimplantitis is an inflammatory condition that causes soft tissue inflammation and gradual bone loss [10]. Its progression has hampered implant survival and success [11-13].
Interleukins and TNFs have been linked to osteonecrosis [14-16]. Pro-inflammatory cytokines (interleukin 1 [IL 1 ], IL 6, IL 8, TNF, and interferon gamma) They activate the immune system, the central nervous system, and hormone release (or repression). Although more research is needed on cytokines, the relevance of their numerous roles is now completely understood [17,18]. While these cytokine-induced reactions are typically beneficial, too much of them may be dangerous. Surplus pro-inflammatory cytokines may cause hypotension, multi-organ failure and death. The body has a sophisticated system of checks and balances to regulate cytokine production and activity, significantly more complex than those that govern endocrine activities [18].
The activation of CD4+ cells produces effector populations that may contribute to the advancement of the inflammatory response [19]. The activation of the adaptive immune response was formerly thought to be mediated by two effector CD4+ T cell subpopulations, namely Th 2 responses, which are prevalent in periodontitis gingiva. Others claim that periodontitis and peri implantitis patients' gingival difficulties have greater Th0 and/or Th1 responses. Since the discovery of TH 17 generating IL-17 in the presence of IL-23, an important cytokine implicated in the growth of the Th17 lineage linked to various immune-related destructive tissue disorders, this paradigm has been updated. The Th17 Pathway and IL-23 in Periodontitis [20,21]. IL-12 induces IFN-expression from T-cells and leads TH cells to become TH1 cells. IFN- also inhibits osseointegration by interfering with the RANKL-RANK signaling pathway [22].
Materials and Methods
A total of 80 subjects (15 peri-implantitis patients and 35 successful implants and 30 healthy controls) were enrolled in this crosssectional study.
The inclusion criteria of patients were required to have an unremarkable medical history, no known allergies, and no metabolic diseases. They also had no history of any antibiotic treatment for the prior 3 months, no use of antiinflammatory drugs in the 6 months preceding the beginning of the study and no radiographic evidence of periodontal bone loss after a fullmouth radiographic periapical examination. The periodontal health of the patients was also evaluated using the plaque index (PI), gingival index (GI), pocket depth (PD) and bleeding on probing (BOP) by a dentist.
Subjects were grouped into 3 groups: group1 consisted of 15 patients (9 males and 5 females, mean age 39 years) with peri-implantitis patients, group 2 consisted of 35 Subject with working Osseo integrated implants i.e. successful implants, (21 males and 15 females, mean age 47 years)and group 3 consisted of 30 Subject randomly taken healthy control adult subjects (19 males and 11 females, mean age 41 years). The study protocol and informed consent forms were approved by the Faculty of Medicine, University of Baghdad.
Sample collection
Patients were scheduled for sample collection during the morning hours that is, from 9 AM to 11 AM. The patients were instructed to refrain from food and vigorous oral hygiene measures 90 minutes prior to sample collection. To avoid salivary contamination, the selected sites were rinsed with water, isolated by cotton rolls, and dried with a gentle air spray. The fluid sample was collected from the test groups by standardized absorbent paper strips (Perio Paper). The supragingival plaque was removed with dry gauze, and a standardized paper strip was inserted 1-2 mm into the sulcus. The strip was held in place for 30 seconds. Strips contaminated by blood were excluded from the sampling group. After 30 seconds of sampling time, paper strips were immediately placed in sterile Eppendorf tubes containing 0.5 ml preservative (PBS) then centrifuged at (3000) rpm for ten minutes, and carefully wrapped to be stored in −80°C until laboratory analysis [23]. Enzyme-linked immunosorbent assay (ELISA) kits were used for analysis and detection of IL- 23 and IL-12 in the patient their peri-implant sulcular fluid specimens.
Statistical analysis
SPSS version 26, Microsoft Excel 2010, Using normality tests, the present study's data was carefully examined to determine if it was parametric or non-parametric. As a result, appropriate statistical tests were employed. Oneway ANOVA and LSD were achieved to evaluate considerable differences. ROC data and person correlation were used to assess the applied parameters as perfect or excellence markers for the studied case together with their association as (v. strong, strong, moderate or weak negatively or positively).
Results
Fifteen patients with Peri-implantitis were rolled in this study, the age of patients with periimplantitis ranged between (20-70) years with a mean of (39.93 ± 8.21) years. Furthermore, there was a significant male's predominance (61.0%) among the patient's group, no statistically significant differences (P=0.888) in age or gender existed between patients and controls. as shown in Table 1.
| Gender | Study groups | Total | P. value | |||
|---|---|---|---|---|---|---|
| Healthy control No. (%) | Implant successful No. (%) | Peri-Implantitis No. (%) | ||||
| Female | 11 (36.7)% | 15 (41.7)% | 5 (35.7%) | 31 (38.8)% | 0.888NS | |
| Male | 19 (63.3)% | 21 (58.3)% | 9 (64.3%) | 49 (61.3)% | ||
| Total | 30 (100.0)% | 36 (100.0)% | 14 (100.0)% | 80 (100.0)% | ||
| Age | Mean ± SD | 41.67 ± 13.15 | 47.33 ± 9.95 | 39.93 ± 8.21 | 43.91 ± 11.33 | |
| Range | (24-69) | (27-66) | (28-56) | (24-69) | ||
Table 1: Demographic data of patients and Successful implant, healthy control.
PISF levels of IL-23 (pg\ml) in healthy subjects, successful implant and patients with periimplantitis
The peri-implant sulcular fluid level of IL-23 in 15 patients with peri-implantitis was compared to the healthy subject as the control group, and in the successful implant group. PISF level of IL-23 showed highly significant increase (P=0.0001) in the group of patients having periimplantitis (609.056 ± 17.080) in comparison with both healthy control and successful implant group (119.947 ± 13.033), (143.438 ± 12.284) respectively, as illustrated in the Tables 2 and Table 3 and Figure 1.
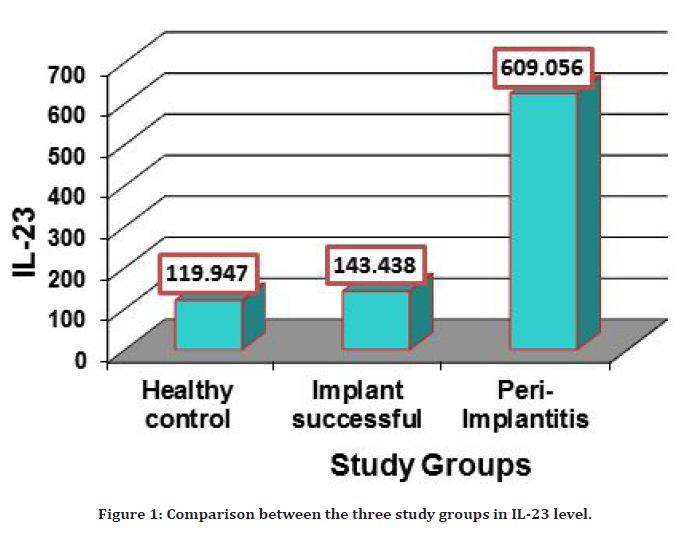
Figure 1. Comparison between the three study groups in IL-23 level.
| Peri-implantitis patients | Healthy Control | P value | |||
|---|---|---|---|---|---|
| Number | N=15 | N=30 | - | ||
| peri-implant sulcular fluid level of IL 23 (pg/ml) | Mean | SE | Mean | SE | 0.0001 |
| 609.056 | ± 17.080 | 119.947 | ± 13.033 | ||
Table 2: Comparison of IL-23 levels (pg/ml) in PISF between healthy subjects and patients with peri-implantitis.
| Number | Peri-implantitis patients | successful implant | P value | ||
|---|---|---|---|---|---|
| N=15 | N=35 | - | |||
| peri-implant sulcular fluid level of IL 23(pg/ml) | Mean | SE | Mean | SE | 0.0001 |
| 609.056 | ± 17.080 | 143.438 | ± 12.284 | ||
Table 3: Comparison of IL-23 levels (pg/ml) in PISF between successful implant group and patients with peri-implantitis.
PISF levels of IL-12 (pg\ml) between healthy subjects, successful implant and patients with periimplantitis
The PISF level of IL-12 detected in 35 patients with Peri-Implantitis and compared to the healthy subjects as the control group, IL-12 level was increased significantly (P=0.0001) in groups of Peri-Implantitis patients (255.485 ± 10.828 pg/ml) in comparison with (154.443 ± 8.263) in the control group as shown in Table 4 and Figure 2.
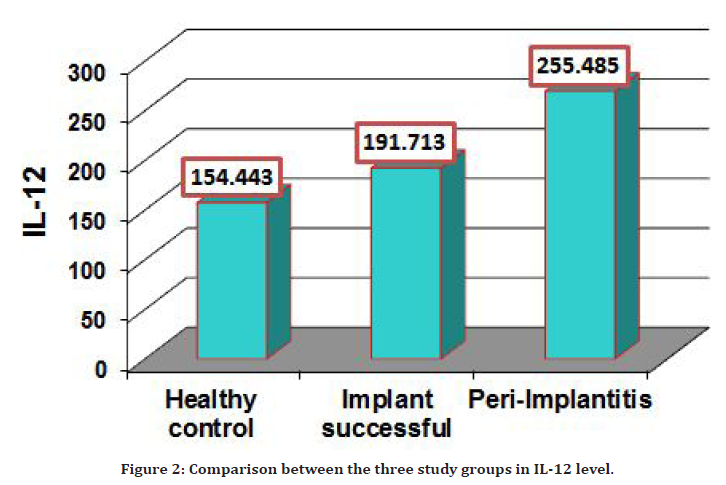
Figure 2. Comparison between the three study groups in IL-12 level.
| Number | Peri-implantitis patients | Healthy Control | P. value | ||
|---|---|---|---|---|---|
| N=15 | N=30 | - | |||
| peri-implant sulcular fluid level of IL 12(pg/ml) | Mean | SE | Mean | SE | 0.0001 |
| 255.485 | ± 10.828 | 154.443 | ± 8.263 | ||
| One way ANOVA | |||||
Table 4: Comparison of IL-12 levels (pg/ml) in PISF between healthy subjects and patients with peri-implantitis.
Also the PTSF level of IL-12 was significantly increased (P=0.0001) in peri-implantitis patients group (255.485 ± 10.828pg/ml) in comparison with (191.713 ± 7.788) successful implant group pg/ml.
The three study groups showed a slight difference level in IL-12 the three study groups show slight difference level in IL-12 as illustrated in Table 5 and Figure 2.
| Number | Peri-implantitis patients | Successful implant | P value | ||
|---|---|---|---|---|---|
| N=15 | N=35 | - | |||
| peri-implant sulcular fluid level of IL 12(pg/ml) | Mean | SE | Mean | SE | 0.0001 |
| 255.485 | ± 10.828 | 191.713 | ± 7.788 | ||
| One way ANOVA | |||||
Table 5: Comparison of IL-12 levels (pg/ml) in PISF between successful implant group and patients with peri-implantitis.
Correlation between the PISF levels of IL-12 and IL- 23 in the three study groups
The results in Table 6 and Figure 3, summarize the correlation between IL-12 and IL-23 in PISF among Healthy control and successful implant groups which were revealed non-significant inverse correlation (r=-0.12)(P=0.503),(r=-0.10) (P=0.546) respectively. In the peri-implantitis patients a statistic significant difference was observed in the correlation between IL-12 and IL-23, indicating a positive relation(r=0.61) (P=0.011).
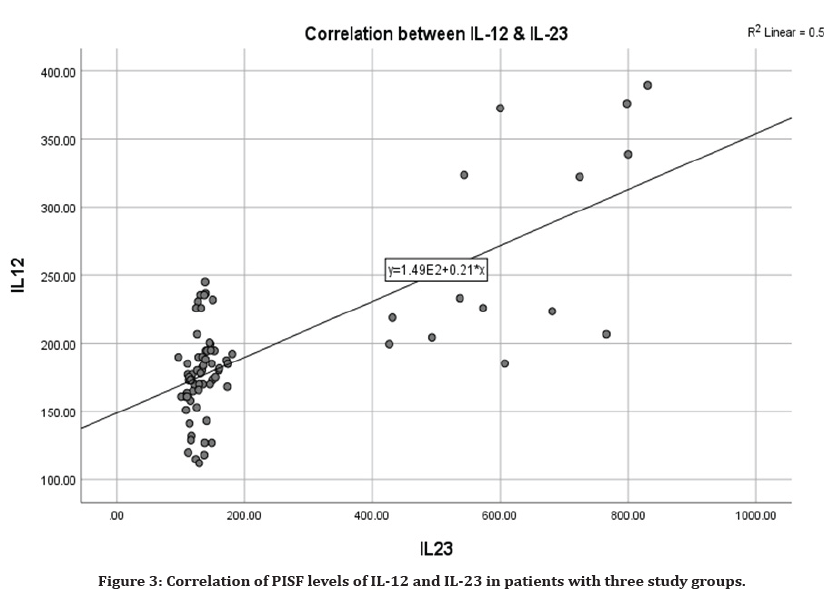
Figure 3. Correlation of PISF levels of IL-12 and IL-23 in patients with three study groups.
| Groups | Correlation coefficient-r between IL-12 and IL-23 | P-value |
|---|---|---|
| Healthy control | -0.12 NS | 0.503 |
| Implant successful | -0.10 NS | 0.546 |
| Peri-Implantitis | 0.61 ** | 0.011 |
| ** (P=0.01), NS: Non-Significant | ||
Table 6: Correlation coefficient between IL-12 and IL-23 in the three study groups.
Discussion
Peri-implantitis: is a pathological condition occurring in tissues around dental implants, characterized by inflammation in the periimplant connective tissue and progressive loss of supporting bone. the exact causes for the development of peri-implant mucositis to peri-implantitis is not clear . The onset of periimplantitis may occur early during follow-up and the disease progresses in a non-linear and accelerating pattern [24].
PISF levels of IL-23 (pg\ml) in healthy subjects, successful implant and patients with periimplantitis.
PISF is a physiological fluid and an inflammatory exudate originating from the gingival plexus of blood vessels in the gingival corium, The composition of PISF can potentially be used to detect subclinical alterations in tissue metabolism, inflammatory-cell recruitment, inflammatory mediators and biomarkers and connective tissue remodeling [25].
It is well known that IL-23 its pro-inflammatory properties. Its ability to potently enhance the expansion of T helper type 17 (Th17) indicates its responsibility for many of the inflammatory autoimmune responses and chronic infections, in addition, different antigens can stimulate IL- 23 production and some other cytokines [26].
In the present study, the levels of IL-23 were determined in PISF obtained from three groups – healthy subjects, patients with Peri-implantitis, and those with successful implants – using an ELISA kit. The study revealed that there was a highly significant increase in PISF level of IL-23 in the patients group having peri-implantitis (P=0.0001) in comparison with both healthy control and successful implant groups. The high level of IL-23 and related loss of its protective effect on the gingival tissue may be explained due to the dysregulation of the IL-23/IL-17 pathway which occur during the progress the peri-implant disease, however, very little data is known about the exact reason for this dysregulation, which warrants further investigations.
Unfortunately, no previous studies concerning peri-implantitis are available to compare with, however, our result was compatible with previous findings detected by Lester et al. [27] who found a significant elevation in the level of IL-23 in GCF and serum among patients with periodontitis, in addition, they reported the association of the IL- 23 concentration with increased progression of Periodontitis (PPD).
Other studies agree with previous study by Vernal et al. [28], Cardoso et al. [29], Ohyama et al. [30], Rohaninasab et al. [31] and Althebeti et al. [32], who found significant elevation of IL- 17 and IL-23 in cases with moderate to severe chronic periodontitis particularly in regions adjacent to the bone loss and they concluded that the activity and stimulation of Th17 cells could be observed in periodontal inflammatory lesions.
In addition, several studies than by Schenkein et al. [33]; Borch, et al. [34] and Graves et al. [35] on periodontal inflammation support our study, they stated higher levels of IL-17, IL-23 cytokines in serum and GCF samples.
Where they said that The enhanced systemic inflammatory response caused by local cytokine generation leads to periodontal tissue destruction.
Our result agrees with Chen et al. [36] and Ju et al. [37] who reported that IL-23 boosted osteoclast development by directly increasing RANK in precursor cells and indirectly increasing RANKL expression on CD4 T cells leading to bone resorption
While Dutzan et al. [38] examined the mRNA of IL-23 and showed a significant overexpression of this cytokine in periodontal diseaseaffected tissues compared to healthy gingival tissues. Jafarzadeh et al. [39] assumed that the raised IL-23 levels might add to the systemic inflammatory burden a predisposing factor to cardiovascular complications, also may lead to a lack of osseointegration and bone loss or implant failure [40-42].
Other investigators as Himani et al. [43], Cifcibasi et al. [44] described a decrease in IL-23 after periodontal therapy, other studies as Duarte, et al. [45], Santos et al. [46], de Ribeiro et al. [47], Reports conflicting results they found almost no change in the levels of IL-23 after therapy.
As the IL-23 level in the gingival crevicular fluid is directly proportional to relative attachment loss , it may be considered as an active factor in guiding and progression of periodontal disease, thus can be regarded as a marker for the inflammatory activity in the periodontium during the process of tissue destruction [43]. It can be postulated that the greater the extent of peri-implant tissue destruction, the higher the concentration of IL-23 in the gingival crevicular fluid signifies the pro-inflammatory role of this cytokine in such diseases.
PISF levels of IL-12 (pg\ml) in healthy subjects, successful implant and patients with periimplantitis
Several studies reported that immunologic factors particularly interleukins and TNFs might influence the development of osteonecrosis [14,48,49]. The usefulness of IL-12 as a marker of peri-implant disease including mucositis and peri-implantitis has been investigated, this cytokine has pro-inflammatory functions and induces bone reabsorption. Nevertheless, it Promotes other cytokine production, predominantly of IFN-γ, from NK and T cells, it functions as a growth factor for activation of these cells, improves the cytotoxic activity of NK cells, and favours cytotoxic T lymphocyte formation [50-52].
BMMSC osteogenesis is inhibited by IL-12. IL-12 and IFN- are effective osteoclast differentiation inhibitors [53-55]. In BMMs and RAW264.7 cells, IL12 modulates NFATc1 expression and suppresses RANKL's osteoclast genic activity [56]. IL-12 also increases TNF-induced osteoclast apoptosis through Fas/Fas ligand interaction [57].
In the present study, the mean level of IL-12 in PISF was obviously higher in the peri-implantitis group when compared to healthy controls and successful implant groups. The high level of this cytokine in patients may be explained since the IL-12 is considered as a pro-inflammatory mediator involved in the inflammatory response. However, IL-12 alone does not activate the osteoclast activity and may inhibit the activity of RANKL. This means that level of IL-12 has no direct and strong effect on inflammation-induced bone loss.
Our result agrees with previous findings reported by Spyrou et al. [58]; Liskmann et al. [59], Konttinen et al. [60] and Duarte et al. [61] that showed significant elevation in level of IL- 12 in PISF and GCF among patients with periimplantitits as compared to successful implant groups and healthy controls, they also described the significant role of this interleukin in gingival inflammation and its unique protective character against bone loss.
Our result disagree with other studies conducted by Mengel et al. [62], Salcetti et al. and Campos et al. which reported no significant relationship between peri-implant disease and early implant loss concerning the level of IL-12 .
Correlation between the PISF levels of IL-12 and IL- 23 in the three study groups
Based on data in this study the correlation between IL-12 and IL-23 in PISF among periimplantitis patient groups were revealed a significant and positive correlation and in the three study groups. Unfortunately, there is no study evaluating the correlation between these two cytokines in this context. However, this correlation was consistent with Xu et al. who detect the effects of IL-12 and IL-23 on bone mass maintenance and formation in mice, they stated that These two interleukins share cytokine subunits and receptor chains, in which IL-23 is formed by two p19 and p40 subunits, The later subunit is which placed between IL-12 and IL-23, have different functions in autoimmune diseases, cancer and pathologies associated with bone disorders and osteoarthritis. And stated that the combination of IL-12, IL-23 and other cytokines specially IL-17 and IFN-γ will promoted osteoclast differentiation, suggesting that the osteoclast-inducing ability of IL-23 should be significantly stronger than that of IL-12, IL-17 and IFN-γ . Our result can be explained by the strong ability of IL-23 to promote osteoclast formation and differentiation, especially when combined and assisted by IL-12. In addition to IL-17 this strengthens osteoclast ability to induce bone loss.
Conclusion
In summary, the IL-23 can act as a biomarker to predict diseases and treatment efficacy and can be used as an effective therapeutic drug to combat inflammatory bone disorders. Targeting IL-12 can promote tissue repair and protect bone mass, whereas IL-12 deletion paradoxically mediates bone loss.
References
- Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med 2004; 350:1422-9.
- Brånemark PI. The osseointegration book: From calvarium to calcaneus. Quintessence Publishing Company 2005.
- Hadi SA, Ashfaq N, Bey A, et al. Biological factors responsible for failure of osseointegration in oral implants. Biol Med 2011; 3:164-170.
- Esposito M, Hirsch JM, Lekholm U, et al. Biological factors contributing to failures of osseointegrated oral implants,(I). Success criteria and epidemiology. Eur J Oral Sci 1998; 106:527-51.
- Buddula A, Assad DA, Salinas TJ, et al. Survival of dental implants in irradiated head and neck cancer patients: A retrospective analysis. Clin Implant Dent Related Res 2012; 14:716-22.
- Lechner J, Noumbissi S, von Baehr V. Titanium implants and silent inflammation in jawbone-a critical interplay of dissolved titanium particles and cytokines TNF-a and RANTES/CCL5 on overall health. EPMA J 2018; 9:331-43.
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol 2007; 127:514-25.
- Buddula A. Bacteria and dental implants: A review. J Dent Implants 2013; 3:58.
- Pokrowiecki R, Mielczarek A, Zareba T, et al. Oral microbiome and peri-implant diseases: Where are we now. Ther Clin Risk Manag 2017; 13:1529.
- Ramanauskaite A, Juodzbalys G. Diagnostic principles of peri-implantitis: A systematic review and guidelines for peri-implantitis diagnosis proposal. J Oral Maxillofac Res 2016; 7.
- Koyanagi T, Sakamoto M, Takeuchi Y, et al. Analysis of microbiota associated with peri-implantitis using 16S rRNA gene clone library. J Oral Microbiol 2010; 2:5104.
- Papathanasiou E, Finkelman M, Hanley J, et al. Prevalence, etiology and treatment of peri-implant mucositis and peri-implantitis: A survey of periodontists in the United States. J Periodontol 2016; 87:493-501.
- Pimentel SP, Shiota R, Cirano FR, et al. Occurrence of peri-implant diseases and risk indicators at the patient and implant levels: A multilevel cross-sectional study. J Periodontol 2018; 89:1091-100.
- Mont MA, Jones LC, Einhorn TA, et al. Osteonecrosis of the femoral head: potential treatment with growth and differentiation factors. Clin Orthop Related Res 1998; 355:S314-35.
- Weitzmann MN, Pacifici R. The role of T lymphocytes in bone metabolism. Immunol Rev 2005; 208:154-68.
- Sato K, Takayanagi H. Osteoclasts, rheumatoid arthritis, and osteoimmunology. Curr Opin Rheumatol 2006; 18:419-26.
- Rübe CE, Uthe D, Schmid KW, et al. Dose-dependent induction of transforming growth factor β (TGF-β;) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiation Oncol Biol Phys 2000; 47:1033-42.
- Zhang JM, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin 2007; 45:27.
- Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res 2010; 20:4-12.
- Zhang X, Xu H, Lin J, et al. Peritoneal fluid concentrations of interleukin-17 correlate with the severity of endometriosis and infertility of this disorder. Int J Obstetr Gynaecol 2005; 112:1153-5.
- Chi W, Yang P, Li B, et al. IL-23 promotes CD4+ T cells to produce IL-17 in Vogt-Koyanagi-Harada disease. J Allergy Clin Immunol 2007; 119:1218-24.
- Jin Q, Cirelli JA, Park CH, et al. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J Periodontol 2007; 78:1300-13088.
- Bhardwaj S, Prabhuji ML. Comparative volumetric and clinical evaluation of peri-implant sulcular fluid and gingival crevicular fluid. J Periodont Implant Sci 2013; 43:233-42.
- Mann S. A model for studying peri-implantitis in vitro. University of Glasgow (United Kingdom) 2011.
- Barros SP, Williams R, Offenbacher S, et al. Gingival crevicular fluid as a source of biomarkers for periodontitis. Periodontol 2016; 70:53-64.
- Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Investigat 2006; 116:1218-22.
- Lester SR, Bain JL, Johnson RB, et al. Gingival concentrations of interleukin-23 and-17 at healthy sites and at sites of clinical attachment loss. J Periodontol 2007; 78:1545-50.
- Vernal R, Dutzan N, Chaparro A, et al. Levels of interleukin-17 in gingival crevicular fluid and in supernatants of cellular cultures of gingival tissue from patients with chronic periodontitis. J Clin Periodontol 2005; 32:383-9.
- Cardoso L, Herman BC, Verborgt O, et al. Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Mineral Res 2009; 24:597-605.
- Ohyama H, Kato-Kogoe N, Kuhara A, et al. The involvement of IL-23 and the Th17 pathway in periodontitis. J Dent Res 2009; 88:633-638.
- Rohaninasab M, Sattari M, Abedi H, et al. The effect of periodontal therapy on IL-17 and IL-23 in gingival crevicular fluid (GCF) of patients with severe periodontitis. Caspian J Dent Res 2013; 2:32-38.
- Althebeti GR, Elfasakhany FM, Talla EA. Evaluation of interleukin-23 in periodontal health and disease. Int J Health Sci Res 2018; 8:226-32.
- Schenkein HA, Koertge TE, Brooks CN, et al. IL-17 in sera from patients with aggressive periodontitis. J Dent Res 2010; 89:943-947.
- Borch TS, Løbner M, Bendtzen K, et al. Decreased interleukin-2 responses to Fusobacterium nucleatum and Porphyromonas gingivalis in generalized aggressive periodontitis. J Periodontol 2009; 80:800-807.
- Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol 2008; 79:1585-91.
- Chen Z, O’Shea JJ. Th17 cells: A new fate for differentiating helper T cells. Immunol Res 2008; 41:87-102.
- Ju JH, Cho ML, Moon YM, et al. IL-23 induces receptor activator of NF-κB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J Immunol 2008; 181:1507-18.
- Dutzan N, Vernal R, Vaque JP, et al. Interleukin-21 expression and its association with proinflammatory cytokines in untreated chronic periodontitis patients. J Periodontol 2012; 83:948-54.
- Jafarzadeh A, Esmaeeli-Nadimi A, Nough H, et al. Serum levels of interleukin (IL)-13, IL-17 and IL-18 in patients with ischemic heart disease. Anatolian J Cardiol 2009; 9.
- Salcetti JM, Moriarty JD, Cooper LF, et al. The clinical, microbial, and host response characteristics of the failing implant. Int J Oral Maxillofac Implants 1997; 12.
- Campos MI, dos Santos MC, Trevilatto PC, et al. Interleukin-2 and interleukin-6 gene promoter polymorphisms, and early failure of dental implants. Implant Dent 2005; 14:391-8.
- Nowzari H, Botero JE, DeGiacomo M, et al. Microbiology and cytokine levels around healthy dental implants and teeth. Clin Implant Dent Related Res 2008; 10:166-173.
- Himani GS, Prabhuji ML, Karthikeyan BV. Gingival crevicular fluid and interleukin-23 concentration in systemically healthy subjects: Their relationship in periodontal health and disease. J Periodont Res 2014; 49:237-245.
- Cifcibasi E, Koyuncuoglu C, Ciblak M, et al. Evaluation of local and systemic levels of interleukin-17, interleukin-23, and myeloperoxidase in response to periodontal therapy in patients with generalized aggressive periodontitis. Inflammation 2015; 38:1959-68.
- Duarte PM, da Rocha M, Sampaio E, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: A pilot study. J Periodontol 2010; 81:1056-63.
- Santos VR, Ribeiro FV, Lima JA, et al. Partial-and full-mouth scaling and root planing in type 2 diabetic subjects: a 12-mo follow-up of clinical parameters and levels of cytokines and osteoclastogenesis-related factors. J Periodont Res 2012; 47:45-54.
- Vieira Ribeiro F, de Mendonça AC, Santos VR, et al. Cytokines and bone-related factors in systemically healthy patients with chronic periodontitis and patients with type 2 diabetes and chronic periodontitis. J Periodontol 2011; 82:1187-96.
- Sato K, Suematsu A, Okamoto K, et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med 2006; 203:2673-82.
- Schierano G, Bellone G, Cassarino E, et al. Transforming growth factor-β and interleukin 10 in oral implant sites in humans. J Dent Res 2003; 82:428-32.
- Liskmann S, Vihalemm T, Salum O, et al. Correlations between clinical parameters and lnterleukin-6 and lnterleukin-10 levels in saliva from totally edentulous patients with peri-implant disease. Int J Oral Maxillofac Implants 2006; 21.
- López Carriches C, Martínez González JM, Donado Rodríguez M. Variations of interleukin-6 after surgical removal of lower third molars. Med Oral Patol 2006; 11E520-E526.
- Horwood NJ, Elliott J, Martin TJ, et al. IL-12 alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol 2001; 166:4915-21.
- Nagata N, Kitaura H, Yoshida N, et al. Inhibition of RANKL-induced osteoclast formation in mouse bone marrow cells by IL-12: Involvement of IFN-possibly induced from non-T cell population. Bone 2003; 33:721-32.
- Shanmugarajan S, Kawanabe N, Koide M, et al. IL-12 stimulates the osteoclast inhibitory peptide-1 (OIP-1/hSca) gene expression in CD4+ T cells. J Cellular Biochem 2009; 107:104-11.
- Amcheslavsky A, Bar-Shavit Z. Interleukin (IL)-12 mediates the anti-osteoclastogenic activity of CpG-oligodeoxynucleotides. J Cell Physiol 2006; 207:244-50.
- Kitaura H, Nagata N, Fujimura Y, et al. Effect of IL-12 on TNF-a-mediated osteoclast formation in bone marrow cells: Apoptosis mediated by Fas/Fas ligand interaction. J Immunol 2002; 169:4732-47388.
- Spyrou P, Papaioannou S, Hampson G, et al. Cytokine release by osteoblast-like cells cultured on implant discs of varying alloy compositions. Clin Oral Implants Res 2002; 13:623-630.
- Konttinen YT, Ma J, Lappalainen R, et al. Immunohistochemical evaluation of inflammatory mediators in failing implants. Int J Periodont Restor Dent 2006; 26:135.
- Duarte PM, De Mendonça AC, Máximo MB, et al. Differential cytokine expressions affect the severity of peri-implant disease. Clin Oral Implants Res 2009; 20:514-520.
- Mengel R, Stelzel M, Hasse C, et al. Osseointegrated implants in patients treated for generalized severe adult periodontitis. An interim report. J Periodont 1996; 67:782-787.
- Xu J, Li J, Hu Y, et al. IL-23, but not IL-12, plays a critical role in inflammation-mediated bone disorders. Theranostics 2020; 10:3925.
- Lacativa PG, Farias ML. Osteoporosis and inflammation. Arq Bras Endocrinol Metabol 2010;54:123-32.
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Indexed at, Google Scholar, Cross Ref,
Author Info
Ehab Qasim Talib* and Ghada Ibrahim Taha
Department of Basic Sciences, College of Dentistry, University of Baghdad, IraqReceived: 04-Mar-2022, Manuscript No. JRMDS-22-51575; , Pre QC No. JRMDS-22-51575 (PQ); Editor assigned: 07-Mar-2022, Pre QC No. JRMDS-22-51575 (PQ); Reviewed: 21-Mar-2022, QC No. JRMDS-22-51575; Revised: 25-Mar-2022, Manuscript No. JRMDS-22-51575 (R); Published: 31-Mar-2022
