Research Article - (2023) Volume 11, Issue 1
Isolation and Characterization of Galantamine Present in the bulb of Narcissus jonquilla L. plant cultivated in Iraq
Usama Hasan Abdul Wahab1*, Eman Saadi Salah2 and Ammar A Razzak Mahmood3
*Correspondence: Usama Hasan Abdul Wahab, Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Anbar, Ramadi, Iraq, Email:
Abstract
Galantamine was isolated from the bulb part of Narcissus jonquilla L. plant cultivated in Iraq. The compound was identified by different chemical analysis like: Fourier Transforms Infrared spectra (FTIR), High Performance Liquid Chromatography (HPLC) and mass spectroscopy and 1H-NMR.
Keywords
Narcissus jonquilla, Galantamine, FTIR, HPLC, MASS, TsupH-NMR
Introduction
The Amaryllidaceae are a family of herbaceous, mainly perennial and bulbous (rarely rhizomatous) flowering plants in the monocot order asparagales [1,2]. The family takes its name from the genus Amaryllis and is commonly known as the amaryllis family [3,4]. The leaves are usually linear and the flowers are usually bisexual and symmetrical, arranged in umbels on the stem [5,6]. The petals and sepals are undifferentiated as tepals, which may be fused at the base into a floral tube. Some also display a corona. Allyl sulfide compounds produce the characteristic odor of the onion subfamily (allioideae) [7,8]. Narcissus is a genus of predominantly spring flowering perennial plants of the amaryllis family, amaryllidaceae. Various common names including Daffodil, Narcissus and Jonquil are used to describe all or some members of the genus [9].
Narcissus has conspicuous flowers with six petals like tepals surmounted by a cup or trumpet shaped corona [10]. The flowers are generally white and yellow (also orange or pink in garden varieties), with either uniform or contrasting colored tepals or corona.
Acetylcholinesterase inhibitory activity amaryllidaceae alkaloids and narcissus extracts were used in Alzheimer’s disease as inhibitors of acetylcholine esterase [11,12]. Antibacterial and antifungal effects the antimicrobial activity of the ethanolic extract of the aerial parts of N. jonquilla was investigated against gram negative and gram positive bacteria [13,14].
Antiviral effects and alkaloid extract of the N. jonquilla, inhibits the purified DNA polymerase from avian myeloblastosis virus by a mechanism differed from that of other known inhibitors. Anti-plasmodium effects the activity of amaryllidaceae alkaloids and the extracts of Amaryllidaceae plants was studied in vitro against a chloroquine resistant (K1) strain of Plasmodium falciparum [15]. Cardiovascular effects the ethanol extract of the bulbs of N. jonquilla were studied for cardiovascular effects using in vivo and in vitro preparations of normotensive rats in the presence and absence of various (α, β-adrenergic, cholinergic, ganglionic, histaminergic, enzyme conversion inhibitor and calcium channel blockers) [16]. Immunomodulatory effects the immune modulation of N. jonquilla lectin on the induction of gene expression of cytokines in the mouse was studied, using specific cytokine primers, total RNA isolated from mouse splenocytes and macrophages and reverse transcription polymerase chain reaction. Cytotoxic effects the aerial parts extracts of N. jonquilla possessed in vitro anticancer activity against MCF-7 [17].
Galantamine is used for the treatment of cognitive decline in mild to moderate Alzheimer's disease and various other memory impairments [18,19].
Stigmasterol a plant sterol (phytosterol) is among the most abundant of plant sterols, having a major function to maintain the structure and physiology of cell membranes.
Materials and Methods
Plant materials
The whole plant of N. jonquilla plant family (amaryllidaceae) was collected from the Baghdad city on the farm in Palestine Street during the November, March and April (2020-2021). The plant bulb was cleaned and dried in oven at a temperature (50°C-60°C) for (15-20) mints then these leaves were coarsely powered by mechanical grinder and weighted.
Extraction methods of N . jonquilla
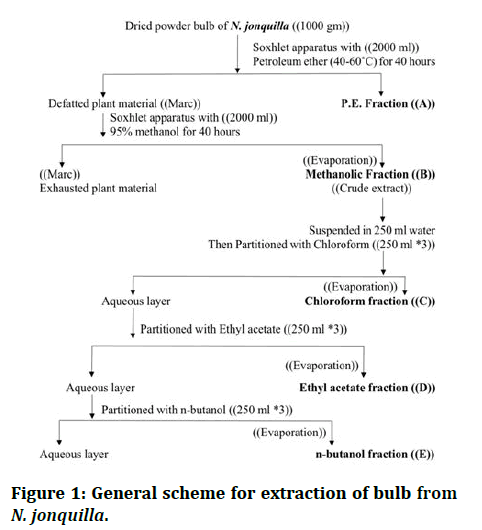
500 grams of the dried powdered bulb of N. jonquilla were extracted with soxhlet apparatus by with volume (1000 ml) of petroleum ether (40°C-60°C) for 48 hours to give fraction (A), then extracted with Soxhlet apparatus again with volume (1000 ml) of 95% methanol for 48 hours to give fraction (B). The methanolic extract (B) was evaporated to dryness by rotary vaporization at 60°C under reduced pressure and then fractionation with Chloroform, Ethyl acetate and nbutanol respectively (250 ml × 3 each) to give fractions (C), (D), (E) and (F) then subjected to identification (Figure 1).
Figure 1: General scheme for extraction of bulb from N. jonquilla.
Isolation and purification of isolated galantamine
Preparative TLC plates: The major fractions chloroform was carried out by using preparative TLC which was performed by using readymade plates of 20 cm × 20 cm, which are coated by silica gel GF 254 layers of 1 mm thickness, (Merck). The fractions chloroform obtained from method of extraction applied as a concentrated solution in a row of spots using capillary tube four times on each plate (the spots should be dried before the next application). The solvent systems (Toluene: Ethyl acetate: Formic acid: Acetic acid) (20:10:10:7.5) was each placed in a glass tank (22.5 cm × 22 cm × 7 cm) and covered with a glass lid and allowed to stand for 45 minutes before use for saturation. The band corresponding to the galantamine standard were scraped out and collected in beaker, mixed with methanol, stirred and left a side for one hour, then filtered. After evaporation of the solvent, the residue obtained was subjected to chromatography with the available reference standard of galantamine using different mobile phases for identification.
Identification of characterization of isolated galantamine
Infrared (IR spectroscopy): IR is a well-developed technique of identifying functional chemical groups in the compounds infrared spectroscopy. The infrared spectroscopy technique involves the study of radiant energy reflection, absorption or transmission from the wavelength 500 cm-1 to 4000 cm-1 area of the electromagnetic spectrum.
The frequency and commonly expressed in wavenumbers is a more widely used calculation. The IR range is normally broken down into 3 areas, namely close IR (12500 cm-1 to 4000 cm-1) medium IR (4000 cm-1 to 300 cm-1) and high IR (400 cm-1 to 800 cm-1) and just medium IR (400 cm-1 to 20 cm-1). Infra-Red (IR) spectra of isolated compounds were recorded in Shimadzu FTIR spectrometer in the range of wave number 500 cm-1 to 4000 cm-1 and operated in the transmittance mode. The mold and press of a pulver containing approximately 1 mg of substance were then used for preparing the thin disk under anhydrous conditions. Within three minutes the spectrum was registered. IR spectroscope technology is a very important tool for identifying several varieties of plant constituents in phytochemical studies as a fingerprint device for evaluating a natural compound. It also helps in the systemic elucidation of new compounds in plants.
HPLC analysis: Qualitative and quantitative estimation of galantamine using HPLC analysis:
HPLC conditions of chloroform fraction:
• Mobile phase: Isocratic: Methanol: DW (80:20).
• Column: SYKAMLC C18-ODS (25 mm × 4.6 mm, 5 μm particle size).
• Sample: Chloroform.
• Standards: Galantamine.
• Flow rate: 1 ml/min.
• Injection volume: 100 μL.
• Injection concentration: 1 mg/ml.
• Detection mode and setting: UV detector at λ 25 4 nm.
Mass Spectrometry (MS)
Is an analytical technique that is used to measure the mass to charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass to charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.
Nuclear Magnetic Resonance spectroscopy (NMR analysis: This analysis was performed for the isolated compounds. The proton NMR spectra were taken by dissolving the sample in Dimethyl Sulfoxide (DMSO) operate on an NMR spectrometer. 1H-NMR is an efficient method for identifying and elucidating the structure of various types of components.
Results and Discussion
Isolation and characterization of isolated galantamine FTIR spectroscopy
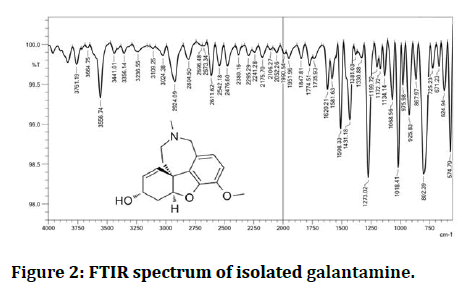
FTIR interpretation of isolated galantamine: The detected absorption bands are 3556 cm-1-3356 cm-1 typical of the O-H stretching when exposed to IR spectroscopic study. The absorption is assumed at 3024 cm-1 C-H stretching of aromatic ring, 2924 cm-1 to =CH, 2804 cm-1 to C-H. As a result of C=C stretching aliphatic, the band is weakened by 1620 cm-1 however, the absorption 1554 cm-1-1508 cm-1 C=C aromatic absorption frequencies also include 1431 cm-1-1381 cm-1 C-H bending CH3 and CH2. The level of absorption at 1273 cm-1 C-O stretching of ether (Figure 2).
Figure 2: FTIR spectrum of isolated galantamine.
HPLC analysis
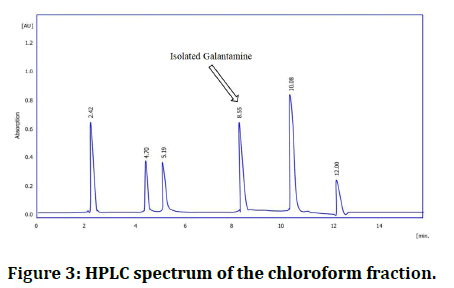
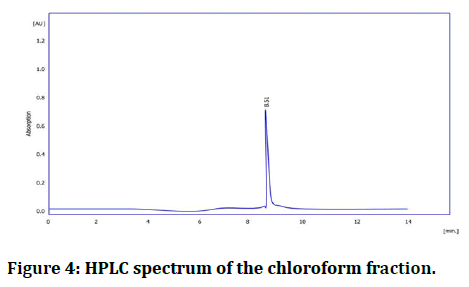
HPLC spectrum of chloroform fraction and isolated galantamine: The retention time for the isolated galantamine was identical to the main peak of the chloroform fraction, and standard reference; more over the peaks isolated galantamine and the standard reference was super imposable (Table 1, Figures 3 and 4).
| Samples | Retention time (Min) |
|---|---|
| Galantamine standard | 8.51 |
| Isolated galantamine | 8.55 |
Table 1: Retention time of isolated compound galantamine and the standard reference.
Figure 3: HPLC spectrum of the chloroform fraction.
Figure 4: HPLC spectrum of the chloroform fraction.
Mass spectrometry analysis
Mass spectrometry is done by ESI: Electron spray ionized method.
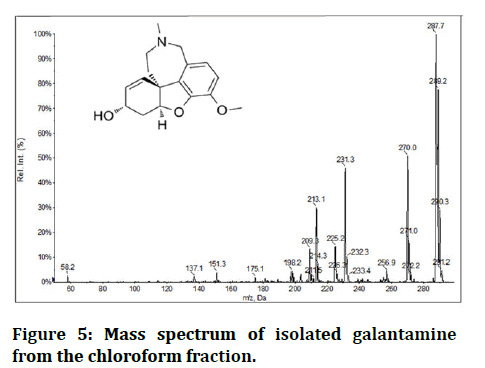
Mass spectrum interpretation of isolated galantamine: MS (ESI) m/z: Calcd. for CI7H21NO3 [M]+ 287.15, found 287.7 (Figure 5).
Figure 5: Mass spectrum of isolated galantamine from the chloroform fraction.
1H-NMR analysis
1H-NMR Interpretation of isolated galantamine
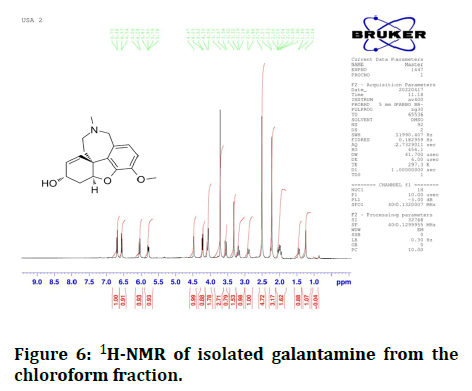
1H-NMR (400 MHz, DMSOd6, δ=ppm): 4.47 (m,1H, H1), 1.96-2.06 (m,2H, H2α, B), 4.23 (m,1H, H3), 4.23 (d,1H, H6), 4.47 (d,1H, H6'), 6.80 (d,1H, ArH8), 6.57 (d,1H, ArH7), 3.72 (s,3H, OCH3), 3.57 (d,1H12α), 3.19 (m,1H, H12β), 2.27 (s, 3H, N-CH3), 2.91 (d,1H, H11β), 1.46 (d,1H, H11α) (Figure 6).
Figure 6: 1H-NMR of isolated galantamine from the chloroform fraction.
Conclusion
Galantamine was isolated from the bulb part of Narcissus jonquilla L. plant cultivated in Iraq. The compound was identified by different chemical analysis like: Fourier Transforms Infrared spectra (FTIR), High Performance Liquid Chromatography (HPLC) and mass spectroscopy and 1H-NMR.
Acknowledgment
The authors are grateful for the research facilities given to us by the Jordan University of science and technology.
References
- Xu Z, Chang L. Amaryllidaceae. Identification and control of common weeds, Springer, 2017; 3:877-889.
- Ka S, Koirala M, Merindol N, et al. Biosynthesis and biological activities of newly discovered Amaryllidaceae alkaloids. Molecules 2020; 25:4901. [Crossref][Googlescholar][Indexed]
- Nair JJ, van Staden J. Pharmacological studies of Crinum, Ammocharis, Amaryllis and Cyrtanthus species of the South African Amaryllidaceae. J South African J Botany 2022; 147:238-244. [Crossref][Googlescholar][Indexed]
- Tallini LR, Andrade JPd, Kaiser M, et al. Alkaloid constituents of the Amaryllidaceae plant Amaryllis belladonna L. J Molecules 2017; 22:1437. [Crossref][Googlescholar][Indexed]
- Voss EG, Reznicek AA. Field manual of michigan flora. University of Michigan Press, 2012. [Crossref][Googlescholar][Indexed]
- Shamso EM, Draz A, Hosni HA, et al. Taxonomic revision of genus Oxalis L. (Oxalidaceae) in the flora of Egypt. J Taeckholmia 2022; 41:56-69. [Crossref][Googlescholar][Indexed]
- de Craene LPR. Floral diagrams: An aid to understanding flower morphology and evolution. Cambridge University Press; 2022. [Googlescholar][Indexed]
- Sabiu S, Madende M, Ajao AA-n, et al. The genus Allium (Amaryllidaceae: Alloideae): Features, phytoconstituents and mechanisms of antidiabetic potential of Allium cepa and Allium sativum. Bioactive food as dietary interventions for diabetes 2019; 137-154. [Crossref][Googlescholar][Indexed]
- Tull D. 5. Poisonous and harmful plants. Edible and useful plants of the Southwest. University of Texas Press 2021; 226-300. [Crossref]
- Azizi K, Konoz E. Chemical analysis of essential oil component, perfume and synthetic essential oil of narcissus and its harmful compounds. Main Group Chem 2022; 21:101-112. [Crossref]
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm 2020; 10:44-53. [Googlescholar]
- Ronsted N, Savolainen V, Mølgaard P, et al. Phylogenetic selection of Narcissus species for drug discovery. Biochem Syst Ecol 2008; 36:417-422. [Crossref][Googlescholar][Indexed]
- Boshra YR, Fahim JR, Hamed ANE, et al. Phytochemical and biological attributes of Narcissus pseudonarcissus L. (Amaryllidaceae): A review. S Afr J Bot 2022; 146:437-58. [Crossref][Googlescholar][Indexed]
- Benedec D, Oniga I, Hanganu D, et al. Sources for developing new medicinal products: Biochemical investigations on alcoholic extracts obtained from aerial parts of some Romanian Amaryllidaceae species. BMC Complement Altern Med 2018; 18:226. [Crossref][Googlescholar][Indexed]
- Nair JJ, van Staden J. The amaryllidaceae as a source of antiplasmodial crinane alkaloid constituents. Fitoterapia 2019; 134:305-313. [Crossref][Googlescholar][Indexed]
- Jin Z. Amaryllidaceae and sceletium alkaloids. Nat Prod Rep 2009; 26:363-381. [Crossref][Googlescholar][Indexed]
- Jin Z, Yao G. Amaryllidaceae and sceletium alkaloids. Nat Prod Rep 2019; 36:1462-1488. [Crossref][Googlescholar][Indexed]
- Metz CN, Pavlov VA. Treating disorders across the lifespan by modulating cholinergic signaling with galantamine. J Neurochem 2021; 158:1359-1380. [Crossref][Googlescholar][Indexed]
- Sharma S. Galantamine delivery for Alzheimer’s disease. Sustainable Agriculture Reviews 43: Pharmaceutical technology for natural products delivery, fundamentals and applications. 2020; 1:131-159. [Googlescholar]
Author Info
Usama Hasan Abdul Wahab1*, Eman Saadi Salah2 and Ammar A Razzak Mahmood3
1Department of Pharmacognosy and Medicinal Plants, College of Pharmacy, University of Anbar, Ramadi, Iraq2Department of Clinical Laboratory Sciences, College of Pharmacy, University of Baghdad, Baghdad, Iraq
3Department of Pharmaceutical Chemistry, College of Pharmacy, University of Baghdad, Baghdad, Iraq
Citation: Usama Hasan Abdul Wahab, Eman Saadi Salah, Ammar A Razzak Mahmood, Isolation and Characterization of Galantamine Present in the Bulb of Narcissus jonquilla L. Plant Cultivated in Iraq, J Res Med Dent Sci, 2023, 11 (01):232-236
Received: 25-Oct-2022, Manuscript No. JRMDS-22-72767; , Pre QC No. JRMDS-22-72767 (PQ); Editor assigned: 28-Oct-2022, Pre QC No. JRMDS-22-72767 (PQ); Reviewed: 11-Nov-2022, QC No. JRMDS-22-72767; Revised: 26-Dec-2022, Manuscript No. JRMDS-22-72767 (R); Published: 23-Jan-2023