Research Article - (2022) Volume 10, Issue 5
Isolation of Melanin Pigment Producing Marine Actinobacterium of Streptomyces Isolated From Marine Sediment Samples and Their Antibacterial Activity
Lakshminarayanan Arivarasu*, Pravalika Arunkumar and Pitchiah Sivaperumal
*Correspondence: Lakshminarayanan Arivarasu, Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, India, Email:
Abstract
Introduction: Melanin is a pigment produced by organisms throughout all domains of life. In the vast majority of studies, melanin has been either chemically synthesized or isolated from animals. Marine organisms are the source of thousands of substances, which also have antibacterial and antifungal effects. Microbial mediated production is the best viable alternative to obtain melanin. This method has the advantage of being scalable and providing a good yield of melanin. Streptomyces is the largest genus of Actinobacteria and the type genus of the family Streptomycetaceae.
Materials and method: The sediment samples were collected from parangipettai, Tamilnadu. The sediments were sundried for 24-48 hrs and turned into a fine powder with mortar and pestle. Isolation of Actinobacteria, Identification of marine actinobacteria, Production and purification of melanin, Chemical analysis of Melanin. Antibacterial activity: The antibacterial activity of melanin was performed with disc diffusion method. Minimum Inhibitory Concentration was also observed.
Results: Presence of actinobacteria from the isolated sediment sample is confirmed by chemotaxis characteristics. The antibacterial activity of the melanin pigment isolated from Streptomyces sample was analysed and found that the minimum inhibitory concentration was more compared to the standard antibacterial agent that is tetracycline.
Conclusion: In the present study, produced melanin pigment was found to have potent antibacterial potential activity. Further characterization and bio active properties should be done in future studies, and more articles in future are yet to come in various properties of melanin pigment.
Keywords
Melanin, Pigment, Marine, Antibacterial activity, Streptomyces, SedimentsIntroduction
Melanin is a pigment produced by organisms throughout all domains of life. In the vast majority of studies, melanin has been either chemically synthesized or isolated from animals [1]. In previous researches DOPA melanin showed a promising activity as an antibacterial natural product against 12 pathogenic bacteria from hospital isolations [2]. Currently, the increasing resistance of microorganisms to antibiotics is a serious problem. Actinomycetes, one among the most colourful microbes, are characterized by the production of various pigments on different natural and synthetic media [3]. Marine organisms are the source of thousands of substances, which also have antibacterial and antifungal effects. Microbial mediated production is the best viable alternative to obtain melanin. This method has the advantage of being scalable and providing a good yield of melanin [4]. Color of melanin ranges from yellow to black and depends on the metabolic pathway which synthesises them. Melanins are used in a wide variety of applications in day-to-day life including cosmetics, optical lenses, pharmaceuticals, and batteries to name some [5-10].
In previous studies three strains among 21 Actinomycetes sp isolates produced a diffusible dark pigment on starch casein an agar medium which was water soluble. That study proved that sugarcane waste can be used for the production of melanin and it (melanin) has potential antibacterial activity [11]. A study suggested that, on the basis of anti-bacterial activity and MIC test of melanin pigment from Streptomyces sp., MVCS13 can be selected as an effective antibacterial agent for ornamental fish culture. (‘Melanin from marine Streptomyces sp. (MVCS13) with potential effect against ornamental fish pathogens of Carassius auratus. In an article published it proved that melanin has an antibacterial, antifungal, antioxidant activity and also it can be used for industrial biotechnological application because microbial melanin can be produced in large scale with low production cost [12]. Also few articles proved that actinobacteria isolated from marine environments have been dominated by Micromonospora, Rhodococcus and Streptomyces species. Recent culture-independent studies have shown that marine environments contain a high diversity of actinobacterial species that are rarely, if at all, recovered by cultivation-based methods [13]. Our team has extensive knowledge and research experience that has translated into high quality publications. The present study proves to be unique as not many articles have been published on the antibacterial effect of melanin isolated from marine sediments. The aim of this study is to extract melanin from marine actinobacterium of streptomyces from marine sediments and to analyse the antibacterial activity [14-17].
Materials and Methods
Isolation of actinobacteria
Isolation and enumeration of actinobacteria were carried out in Kuster’s Agar Medium (KUA) supplemented with 0.5% (W/v) NaCl. To minimize the fungal and bacterial contamination, KUA medium was supplemented with cycloheximide (10 µg/ml) and nalidixic acid (10 µg/ml) respectively. Collected sediment samples were serially diluted and inoculated on KUA medium and incubated at 36°C for 7 days. The colonies were counted and the population density has been expressed as colony forming units per gram (CFU/g) of sediments. Morphologically distinct colonies were selected and pure cultures were obtained.
Identification of marine actinobacteria
Aerial mass colour: The colour of the mature sporulation aerial mycelium was recorded in naked eye. When the aerial mass colour fell between two colours series, both the colours were recorded. If the aerial mass colour of a strain to be studied showed intermediate tints, then also, both the colour series were noted. The media used were yeast extract malt extract agar and inorganic salt starch agar [17,18].
Melanoid pigments: The grouping was made on the production of melanoid pigments (i.e. greenish brown, brownish black or distinct brown, pigment modified by other colours) on the medium [19]. The strains were grouped as melanoid pigment produced (+) and not produced (-). In a few cases, the production of melanoid pigments was delayed or weak, and therefore, it was not distinguishable. This is indicated as variable (V). This test was carried out on the media ISP-1 and ISP-7 (Appendix I), as recommended by the International Streptomyces Project [20].
Reverse side pigments: Reverse side pigment production of the isolate was determined on ISP7 medium [21]. The pigment production was noted as distinctive (+) and not distinctive or none (-). In case, a colour with low chroma such as pale yellow, olive or yellowish brown occured, it was included in the latter group (-).
Soluble pigments: Soluble pigment production of isolate was observed on ISP7 medium. The diffusible pigment production other than melanin was considered positive (+) and not produced (-). The colour was recorded (red, orange, green, yellow, blue and violet).
Spore chain morphology: Spore morphological characters of the strains were studied by inoculating a loopful of one week old cultures into solidified agar medium containing sterile glass slides. The cultures were incubated at 28+2°C and examined periodically for the formation of aerial mycelium, sporophore structure and spore morphology [22].
Chemotaxonomical characteristics
Hydrolysis: Hydrolysis was done for releasing amino acids. Harvested cells of each strain weighing 20 mg (fresh) were placed in an ampo bottle and 1 ml of 6N HCl was added and sealed with an alcohol blast burner [23]. The samples were kept at 121°C for 20 h in a sand bath. The bottles were cooled by keeping them at a room temperature of 28+2°C. Hydrolysis was also done for releasing sugars. Harvested cells of each strain weighing 50 mg (fresh) were placed in an ampo bottle and 1 ml of 0.5 N HCl was added and sealed with an alcohol blast burner. The samples were kept at 110°C for 2 h. The bottles were then cooled by keeping them at a room temperature of 28+2°C.
Thin Layer Chromatography (TLC): Spotting of the whole cell hydrolysates was made carefully on TLC plate using a microliter pipette. Spots were 5-10 mm in diameter. This was done by multiple applications on the same spot of very small portions of the sample, which were dried by a hand dryer [24-28].
Amino acids: Each sample (3 µl) was applied on the baselines of the TLC plate (20 cm x 20 cm). Adjacent to this, 1 µl of DL-diaminopimelic acid (an authentic material mixture of DAP isomers) and 1 µl of amino acetic acid (glycine) were spotted as standards [29]. The TLC plate was developed with the solvent system containing methanol: pyridine: glacial acetic acid: H2O (5: 0.5: 0.125: 2.5 v/v). It took approximately more than 4 h for development. The spots were visualized by spraying with 0.4% ninhydrin solution in water-saturated nbutanol, followed by heating at 100 for 5 min. Spots of amino acids ran faster than DAP. The sample spots were immediately compared with the spots of the standards since spots gradually disappeared in a few hours [30].
Whole-Cell sugars: On a cellulose TLC plate (20 cm x 20 cm), 5 µl of samples was spotted along with 3 µl of sugar solutions as standards on the same plates. Galactose, arabinose, xylose and madurose were the sugars, which were used as standards. The TLC plate was developed with the solvent mixture containing ethyl acetate: pyridine: acetic acid: distilled water (8: 5: 1: 1.5 v/v). The development time was more than 4 h. Spots were visualized by spraying with aniline phthalate reagent (3.25 g of phthalic acid dissolved in 2 ml of aniline and made upto 100 ml with water saturated n-butanol). The sprayed plate was heated at 100 for 4 min. Hexoses appeared as yellowish brown spots and pentoses, as maroon coloured spots [31-34].
Assimilation of carbon source: The ability of the actinobacterial strain in utilizing various carbon compounds as source of energy was studied, following the method recommended by International Streptomyces Project [35]. Chemically pure carbon source certified to be free of admixture with other carbohydrates and contaminating materials were used for this purpose. Carbon sources for this test were Arabinose, Xylose, Inositol, Mannitol, Fructose, Rhamnose, Sucrose and Raffinose. These carbon sources were sterilized by ether sterilization without heating. The media and plates were prepared and inoculated according to the convention of ISP project. For each of the carbon sources, utilization is expressed as positive (+), negative (-), or doubtful (±). In the 'doubtful ' strains, only a trace of growth slightly greater than that of the control was noticed [36-38].
Production and purification of melanin
The ISP-2 medium prepared in sea water was used for the development of inoculum. The 2 ml of spore suspension from inoculums medium was inoculated in the fermentation medium containing 0.65 ml of glycerol, 0.63 gm of Yeast extract, 0.55 gm of glucose, .08 gm of MgSO4.
The 2 ml of spore suspension was inoculated into fermentation medium (ISP7) for 12 days under the agitation for 200 rpm at ambient temperature. Then cell free supernatant was collected by centrifugation at 10,000 rpm for 15 min. The harvested cell free supernatant containing melanin was adjusted to pH 2 with con. HCl and kept at room temperature for 3 hrs. After incubation the suspension was centrifuged at 10000 rpm at 28°C for 20 mins to palletize the melanin pigment. The pellet was washed 3 times with distilled water and dissolved in a phosphate buffer (PH8) [39,40].
Chemical analysis of Melanin
The chemical test was carried out with a little modification. The solubility test for the black pigment was tested by adding 100 µl of melanin pigment in 1 ml of distilled deionized water, 1N HCl, 1M NaOH, absolute ethanol, acetone (warm), chloroform (warm), Phenol and Benzene. The reaction with the following oxidizing agents was also determined by adding 100 µl of melanin to the 1 ml of 30% Hydrogen peroxide (H2O2) solution. The precipitation test was carried out by adding 100 µl of purified melanin to 1 ml of 1% Fecl3 solution and 1 ml of Con. HCl.
Bacterial Suspension: The pathogenic bacterial strains Staphylococcus aureus, Klebsiella pneumoniae and Vibrio cholarae were collected from the Department of Microbiology, Saveetha medical college and hospital, Tamilnadu. The bacterial pathogens were cultured in Muller -Hinton Broth for 24 hr at room temperature. From this bacterial suspension was prepared with saline and the optical density was measured at 600 nm. The concentration of microbial suspension was fixed as 106 CFU/ml. 1 ml of suspension was spread over on Muller Hinton agar plate and incubated for 24 hrs at ambient temperature [41,42].
Antibacterial activity: The antibacterial activity of melanin was performed with a disc diffusion method. Whatman filter paper discs (5 mm) were impregnated with various concentrations (0.5, 1, 1.5, 2, 2.5 and 3 mg/ml) of leaf extract using ethanol and methanol solvent. The inoculated plates were incubated for 24 hr at room temperature and the inhibition zones around the discs were measured. All the results were expressed from an average of three with standard deviation [43].
Minimum Inhibitory Concentration
Minimal Inhibition Concentration of melanin was determined in 5 concentrations (10-5 0 µg/ml/0.001 to 0.1 mg/ml) with blank (extract in Muller Hinton broth). The inoculated bacteria in test tubes are incubated for 24 hr in ambient temperature then the optical density was observed [44-46].
Results and Discussion
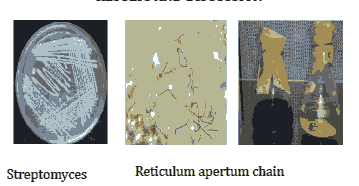
Figure 1: The figure shows the isolation and melanin pigment separation.
Table 1: The table shows the cultural characteristics of Actinobacteria. The colour was found to be grey with morphology of the reticulum apertum chain.
| Positive control - Tetracycline | |
|---|---|
| Color of aerial mycelium | Grey |
| Melanoid pigment | + |
| Reverse side pigment | - |
| Soluble pigment | - |
| Spore chain | RA |
| Arabinose | + |
| Xylose | + |
| Inositol | - |
| Mannitol | ± |
| Fructose | + |
| Rhamnose | + |
| Sucrose | + |
| Raffinose | - |
Melanoid pigment was found and the other pigments like soluble pigment and reverse side pigment were absent. Assimilation of carbon source has shown the presence of xylose and rhamnose among all the carbon compounds mentioned above. These results helped in the isolation of streptomyces species.
The isolated bacteria produced melanoid pigment, but reverse side pigment and soluble pigment was not produced. The reverse side pigment was not distinctive. The streptomyces were grey in colour. It had reticulum apertum chain morphology (Table 1). The cell wall of the bacteria does contain LL-DAP, Glycine but does not contain Arabinose and Galactose. MesoDAP is absent in the bacterium's cell wall. The type of cell wall is type I (Table 2). The pigment produced by actinobacteria of the species Streptomyces proved to be melanin by few characteristics [47,48]. It was black in colour as dark pigments produced by marine organisms are considered to be melanin, if they are dark, insoluble in organic ).
solvents, susceptible to bleaching by oxidising agents and resistant to acidic conditions (Table 5 Antibacterial activity of the extracted melanin was estimated against three bacteria. The zone of inhibition formed against staphylococcus at 50 µg/ml was 6 ± 1.07, 100 µg/ml was 10 ± 1.9, 150 µg/ml was 14 ± 2.3, 200 µg/ml was 20 ± 3.2, 250 µg/ml was 23 ± 2.4 and for 300 µg/ml was 27 ± 2.6. The zone of inhibition formed against pseudomonas was at 50 µg/ml was 7 ± 2.4, 100 µg/ml was 12 ± 1.7, 150 µg/ml was 15 ± 2.4, 200 µg/ml was 19 ± 2.1, 250 µg/ml was 23 ± 2.4 and for 300 µg/ml was 26 ± 2.4. The zone of inhibition formed against vibrio sp. was at 50 µg/ml was 9 ± 1.6, 100 µg/ml was 13 ± 2.5, 150 µg/ml was 17 ± 2.3, 200 µg/ml was 21 ± 2.8, 250 µg/ ml was 24 ± 2.2 and for 300 µg/ml was 28 ± 2.5 (Table 3). The minimum inhibitory concentration for vibrios sp. was observed at 30 µg/ml, 30 µg/ml for pseudomonas and 30 µg/ml for staphylococcus s p (Table 4).
Table 2: The table shows the cell wall characteristics of Actinobacteria. Only LL-DAP and glycine were present and the other cell wall amino acids mentioned above were absent and also both the cell wall cell wall sugars i.e., arabinose and galactose were absent. The cell wall belongs to the first type. This shows an index for Streptomyces species.
| Cellwall amino acids | Cell wall sugar | Index | |||
|---|---|---|---|---|---|
| LL-DAP | MesoDAP | Glycine | Arabinose | Galactose | - |
| + | - | + | - | - | Streptomyces |
Table 3: The table shows the zone of inhibition of the melanin pigment isolated from the streptomyces sample against particular bacteria.
| Melanin pigment µg/ml | Staphylococcus | Pseudomonas | Vibrio. Sp. |
|---|---|---|---|
| 0 | 0 | 0 | |
| 50 | 6 ± 1.07 | 7 ± 2.4 | 9 ± 1.6 |
| 100 | 10 ± 1.9 | 12 ± 1.7 | 13 ± 2.5 |
| 150 | 14 ± 2.3 | 15 ± 2.4 | 17 ± 2.3 |
| 200 | 20 ± 3.2 | 19 ± 2.1 | 21 ± 2.8 |
| 250 | 23 ± 2.4 | 22 ± 2.8 | 24 ± 2.2 |
| 300 | 27 ± 2.6 | 26 ± 2.4 | 28 ± 2.5 |
Table 4: Minimum inhibitory concentration of the melanin pigment isolated from streptomyces in the 3bacterias was compared with the standard antibacterial agent-tetracycline.)
| 0 | 10 | 20 | 30 | 50 | MIC | |
|---|---|---|---|---|---|---|
| Staphylococcus | + | + | + | - | - | 30 µg/ml |
| Tetracycline | + | + | - | - | - | 20 µg/ml |
| Pseudomonas | + | + | + | - | - | 30 µg/ml |
| Tetracycline | + | - | - | - | - | 10 µg/ml |
| Vibrio.sp | + | + | + | - | - | 30 µg/ml |
| Tetracycline | + | - | - | - | - | 10 µg/ml |
Table 5: The table shows the characteristics of pigment obtained from streptomyces species.
The Colour of the pigment was black. The pigment derived showed solubility in phenol which is an organic solvent and insoluble in the other organicsolvents and all the inorganic solvents. It showed precipitation in the presence of FeCl3 and Conc. HCl. The pigment was found to be oxidised with peroxide (H2O2).
| Melanin chemical analysis | |
|---|---|
| Color | Black |
| Solubility in inorganic solvents | |
| H2O (pH 7) | Insoluble |
| 1N HCl | Insoluble |
| 1M NaOH | Insoluble |
| Solubility in organic solvents | |
| Ethanol | Insoluble |
| Chloroform | Insoluble |
| Acetone | Insoluble |
| Benzene | Insoluble |
| Phenol | soluble |
| Precipitation reaction | |
| FeCl3 | Precipitated |
| Conc.HCL | Precipitated |
| Oxidation process | |
| H2O2 | Oxidized |
In previous research, Melanin pigments were extracted from a wide variety of microorganisms including bacteria and fungi. In that study isolation, identification and characterization of melanin from marine actinobacterium (Streptomyces sp. MVCS13) and its potential activities against fish pathogens were investigated [49,50]. Culture conditions and medium composition for the melanin production were optimized. Further, pigment was characterized by UV-vis absorption spectroscopy and FT-IR infrared spectrometry. It has potential antibacterial activity against ornamental fish pathogens Vibrio sp. FPO5 (15 ± 0.01 mm) which is similar to that of the present studies. The Minimum Inhibitory Concentration (MIC) ranges were observed between 18 ± 0.01 and 27 ± 0.03 µg/ml which is lesser than the results obtained in the present study [51]. The previous study suggested that, on the basis of anti-bacterial activity and MIC test of melanin pigment from Streptomyces sp. MVCS13 can be selected as an effective anti-bacterial agent for ornamental fish culture (‘Melanin from marine Streptomyces sp. (MVCS13) with potential effect against ornamental fish pathogens of Carassius auratus. Various articles have been done on isolation of different types of pigments from marine sediments. Mostly extracellular polysaccharides were extracted from actinobacteria is many studies done previously. The marine actinobacteria isolated in this study is streptomyces whereas other studies mostly nocardiopsis .sp were extracted. Similar to the present study, the antimicrobial activities were done in many studies (First Report on Marine Actinobacterial Diversity around Madras Atomic Power Station (MAPS), India, no date) (Physicochemical Profile of Acacia Catechu Bark Extract-An in vitro Stud - International Journal of Pharmaceutical and Phytopharmacological Research, no date) (Awareness of Drug Abuse among Teenagers - International Journal of Pharmaceutical and Phytopharmacological Research, no date (COX2 Inhibitory Activity of Abutilon Indicum - Pharmaceutical Research and Allied Sciences, no date).
Conclusion
In the present study, Streptomyces produced melanin pigment was found to have potent antibacterial potential activity. Further characterization and bio active properties should be done in future studies, and more articles in future are yet to come in various properties of melanin pigment.
Acknowledgements
We sincerely show gratitude to Saveetha Dental College and Hospitals and Saveetha Institute of Medical and Technical Sciences for providing great support for undertaking the study.
Conflicts
The author declared there is no conflict of interest in the present study.
Funding
Sofeene hong kong ltd.
References
- Aathira CM, Geetha RV, Lakshmi T. Knowledge and awareness about the mode of transmission of vector borne diseases among general public. J Pharm Res Int 2020; 87-96. [Googlescholar]
- Ananthan G, Sivaperuma P, Mohamed Hussain S. Antibacterial potential of marine ascidian Phallusia arabica against isolated urinary tract infections bacterial pathogens. Asian J Anim Sci 2011;208-212.
- Arumugam P, George R, Jayaseelan VP. Aberrations of m6A regulators are associated with tumorigenesis and metastasis in head and neck squamous cell carcinoma. Arch Oral Biol 2021;122:105030.
[Googlescholar] [Crossreff] [Pubmed]
- Awareness of Drug Abuse among Teenagers. Int J Pharm Phytopharmacol Res.
- Baskar K, Lakshmi T. Knowledge, attitude and practices regarding HPV vaccination among undergraduate and postgraduate dental students in Chennai. J Pharm Res Int 2020;95-100. [Googlescholar]
- COX2 inhibitory activity of abutilon indicum. Int J Pharm Res Allie.
- Desai C. Exploration of haloarchaea for their potential applications in food industry. J Environ Sci Technol 2020; 4455-4464.
- Dua K. The potential of siRNA based drug delivery in respiratory disorders: Recent advances and progress. Drug Dev Res 2019; 80:714-730.
[Googlescholar] [Crossreff] [Pubmed]
- Duraisamy R. Compatibility of nonoriginal abutments with implants: evaluation of microgap at the implant-abutment interface, with original and nonoriginal abutments. Implant Dent 2019; 28:289-295.
[Googlescholar] [Crossreff] [Pubmed]
- Ezhilarasan D. Oxidative stress is bane in chronic liver diseases: Clinical and experimental perspective. Arab J Gastroenterol 2018; 19:56-64.
[Googlescholar] [Crossreff] [Pubmed]
- Ezhilarasan D, Apoorva VS, Ashok Vardhan N. Syzygium cumini extract induced reactive oxygen species-mediated apoptosis in human oral squamous carcinoma cells. J Oral Pathol Med 2019; 48:115-121.
[Googlescholar] [Crossreff] [Pubmed]
- Ezhilarasan D, Sokal E, Najimi M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreat Dis Int 2018; 17:192-197.
[Googlescholar] [Crossreff] [Pubmed]
- Gheena S, Ezhilarasan D. Syringic acid triggers reactive oxygen species-mediated cytotoxicity in HepG2 cells. Hum Exp Toxicol 2019;38: 694-702.
[Googlescholar] [Crossreff] [Pubmed]
- Gnanavel V, Roopan SM, Rajeshkumar S. Aquaculture: An overview of chemical ecology of seaweeds (food species) in natural products. Aquaculture, 2019; 507:1-6.
- Gomathi AC. Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J Drug Deliv Sci Technol. 2020;55:101376. [Googlescholar]
- Gomathi, M. Green synthesis of silver nanoparticles using Gymnema sylvestre leaf extract and evaluation of its antibacterial activity. S Afr J Chem Eng. 2020. 1-4.
- Jibu RM, Geetha RV, Lakshmi T. Isolation, detection and molecular characterization of Staphylococcus aureus from postoperative infections. J Pharm Res Int 2020; 63-67.
- Joseph B, Prasanth CS. Is photodynamic therapy a viable antiviral weapon against COVID-19 in dentistry? Oral Surg Oral Med Oral Pathol Oral Radiol 2021; 118-119.
[Googlescholar] [Crossreff] [Pubmed]
- Kamala K. Isolation and characterization of biologically active alkaloids from marine actinobacteria Nocardiopsis sp. NCS1. Biocatal Agric Biotechnol 2015. [Googlescholar]
- Crossreff
- Kamala K, Sivaperumal P. Biomedical applications of enzymes from marine actinobacteria. Mar Enzy Biotechnol 2017; 107-123.
[Googlescholar] [Crossreff] [Pubmed]
- Karpinski TM. Marine macrolides with antibacterial and/or antifungal activity. Mar Drugs 2019; 17: 241.
[Googlescholar] [Crossreff] [Pubmed]
- Kishen A, Rajeshkumar S, Preejitha VB. Cynodon dactylon mediated synthesis of selenium nanoparticles and its antimicrobial activity against oral pathogens. Int J Res Pharm Sci 2020; 4152-4156.
- Kumar P. Assessment of potential human health risk due to heavy metal contamination in edible finfish and shellfish collected around Ennore coast, India. Environ Sci Pollut Res Int 2021; 28:8151-8167. .
[Googlescholar] [Crossreff] [Pubmed]
- Kurian NK. A novel melanin producing Pseudoalteromonas lipolytica BTCZ28 isolated from 96 m depth marine sediments. Proceedings 24th Swadeshi Science Congress. 2014.
- Lakshmi T. Antifungal activity of ficus racemosa ethanolic Extract against dermatophytes-an in vitro study. J res med dent sc. 2021;9:191-193.
- Maldonado LA. Diversity of cultivable actinobacteria in geographically widespread marine sediments. Antonie van Leeuwenhoek, 2005; 87:11-18.
- Mangal CSK, Anitha R, Lakshmi T. Inhibition of nitric oxide production and nitric oxide synthase gene expression in LPS activated RAW 264 .7 Macrophages by Thyme oleoresin from Thymus vulgaris. J Young Pharm 2018; 10: 481.
- Manya Suresh LT. Wound healing properties of aloe barbadensis miller-in vitro assay. J Complement Med Res 2020; 11: 30-34.
- Mariona P, Roy A, Lakshmi T. Survey on lifestyle and food habits of patients with PCOS and obesity. J Complement Med Res 2020; 11: 93-98.
- Markov A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther 2021;12:192.
- Melanin from marine Streptomyces sp. (MVCS13) with potential effect against ornamental fish pathogens of Carassius auratus (Linnaeus, 1758). Biocatal Agric Biotechnol. 2014;3:134-141.
- Nandhini NT, Rajeshkumar S, Mythili S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal Agric Biotechnol 2019; 19:101138.
- Nivethitha R. In vitro anticancer effect of Sesamum Indicum Extract. J Complement Med Res 2020;11: 99-105.
- Physicochemical profile of acacia catechu bark extract. an in vitro stud. Int J Pharm Phytopharmacological Res.
- Pushpaanjali G, Geetha RV, Lakshmi T. Knowledge and awareness about antibiotic usage and emerging drug resistance bacteria among dental students. J Pharm Res Int 2020; 34-42.
- 2020; 160:111599.
[Googlescholar] [Crossreff] [Pubmed]
- Rajasekaran, S. Collective influence of 1-decanol addition, injection pressure and EGR on diesel engine characteristics fueled with diesel/LDPE oil blends. Fuel 2020;277:118166.
- Antibacterial potential of marine ascidian Phallusia arabica against isolated urinary tract infections bacterial pathogens
- Raj R K, DE, SR. ß-Sitosterol-assisted silver nanoparticles activates Nrf2 and triggers mitochondrial apoptosis via oxidative stress in human hepatocellular cancer cell line. J Biomed Mater Res 2020; 108:1899-1908.
[Googlescholar] [Crossreff] [Pubmed]
- Ramesh A. Comparative estimation of sulfiredoxin levels between chronic periodontitis and healthy patients - a case-control study. J Periodontol 2018; 89:1241-1248.
- Santhoshkumar J. Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea. S Afr J Chem Eng 2019;29:17-23.
- Saravanan M. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb Pathog 2018;117:68-72.
[Googlescholar] [Crossreff] [Pubmed]
- Sindhu PK. Anorectic drugs: an experimental and clinical perspective-A review. J Complement Med Res 2020;11:106-112. [Googlescholar] [Crossreff] [Pubmed]
- Sivaperumal P. Screening of antibacterial activity of mangrove leaf bioactive compounds against antibiotic resistant clinical isolates. 2010;2:348-353.
- Sivaperumal P. Antimicrobial Peptide from crab haemolypmh of Ocypoda macrocera (Milne Edwards 1852) with reference to antioxidant: A case study. Int J Pharm Pharm Sci 2013;5:719-727. [Googlescholar] [Crossreff] [Pubmed]
- Sivaperumal P, Kamala K, Rajaram R. Bioactive DOPA melanin isolated and characterised from a marine actinobacterium Streptomyces sp. MVCS6 from Versova coast. Nat Prod Res 2015; 29:2117-2121.
[Googlescholar] [Crossreff] [Pubmed]
- Sivaperumal P, Kamala K, Rajaram R. Bioremediation of industrial waste through enzyme producing marine microorganisms. Mar Enzy Biotechnol 2017; 165-179.
[Googlescholar] [Crossreff] [Pubmed]
- Sivaperumal P, Kamala K, Rajaram R. Adsorption of cesium ion by marine actinobacterium Nocardiopsis sp. 13H and their Extracellular Polymeric Substances (EPS) role in bioremediation. Environ Sci Pollut Res 2018; 25:4254-4267.
[Googlescholar] [Crossreff] [Pubmed]
- Sivaperumal P, Kamala K, Rajaram R. Biosorption of long half-life radionuclide of strontium ion (Sr) by marine actinobacteriumnocardiopsissp. Geomicrobiol J. 300-310.
- Syed MH, Gnanakkan A, Pitchiah S. Exploration of acute toxicity, analgesic, anti-inflammatory, and anti-pyretic activities of the black tunicate, Phallusia nigra (Savigny, 1816) using mice model. Environ Sci Pollut Res Int 2021;28:5809-5821.
[Googlescholar] [Crossreff] [Pubmed]
- Vairavel M, Devaraj E, Shanmugam R. An eco-friendly synthesis of Enterococcus sp.-mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ Sci Pollut Res 2020; 27:8166-8175.
[Googlescholar] [Crossreff] [Pubmed]
- Vasanthabharathi V, Lakshminarayanan R, Jayalakshmi S. Melanin production from marine Streptomyces. Afr J Biotechnol 2011; 10:11224-11234.
- Wang Z. Melanin produced by the fast-growing marine bacterium vibrio natriegens through heterologous biosynthesis: characterization and application. Appl Environ Microbiol 2020;
[Googlescholar] [Crossreff] [Pubmed]
Author Info
Lakshminarayanan Arivarasu*, Pravalika Arunkumar and Pitchiah Sivaperumal
Department of Pharmacology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai, IndiaCitation: Pravalika Arunkumar, Lakshminarayanan Arivarasu, Pitchiah Sivaperumal, Isolation of Melanin Pigment Producing Marine Actinobacterium of Streptomyces Isolated from Marine Sediment Samples and Their Antibacterial Activity, J Res Med Dent Sci, 2022, 10(5): 53-60.
Received: 21-Feb-2022, Manuscript No. 44845; , Pre QC No. 44845; Editor assigned: 23-Feb-2022, Pre QC No. 44845; Reviewed: 09-Mar-2022, QC No. 44845; Revised: 22-Apr-2022, Manuscript No. 44845; Published: 04-May-2022
