Research - (2022) Volume 10, Issue 9
Modified Orthodontic Adhesive with Seashell and Zirconium Oxide Nanoparticles and their Effect on Shear bond strength: In-vitro Study
Alaa Gharis Adnan1 and Neam Agha2*
*Correspondence: Neam Agha, Department of Pedodontic, Orthodontic, and Preventive Dentistry, College of Dentistry, Iraq, Email:
Abstract
Aims: the study aims to assess the shear bond strength of modified-heliosit orthodontic adhesive with different concentrations of seashell and zirconium oxide nanoparticles. Materials and methods: Thirty-five sound-extracted human premolars were collected. Heliosit orthodontic adhesive was modified by (8%, 10%, and 12% seashell), and (1%, 3%, and 5% zirconium oxide) nanoparticles. Standard edgewise brackets were bonded to buccal enamel surfaces of the samples of control and six modified adhesive groups. At 24 h after bonding, shear bond strength was measured. Adhesive remnant index was scored under (10X) magnification power of stereomicroscope after de-bonding. The chemical characteristics of orthodontic adhesive material were explored before and after mixing with seashell and zirconium oxide nanoparticles by using FTIR. Results: Seashell groups (8, 10, and 12 %) containing hydroxyapatite nanoparticles had a higher shear bond strength mean value than the control group, (10%) seashell group had the highest mean value, while the least mean value was in (5% ZrO2) group with statistically significant differences between them. About adhesive remnant index, no significant differences were founded among the studying groups. Conclusion: the addition of (10%) seashell nanoparticles had the best performance and improved shear bond strength of heliosit orthodontic adhesive without violation the remnant of adhesive on the buccal enamel surface after brackets de-bonding.
Keywords
Seashell, Biomaterials, Nanoparticles, Shear bond strength, Adhesive remnant index.
Introduction
The bonding technique through adhesive material has been used for the fixation of orthodontic brackets to the labial tooth surface; it has some disadvantages such as the development of white spot lesions, the demineralized enamel around the bracket margins leading to bonding failure [1,2]. With the great development of nanotechnology and Nanomaterials, attention is directed toward the use of nanomaterial’s to reinforce orthodontic adhesive resins and increase their bond strength, and for producing a Nano composite with improved physical and mechanical properties [3]. Different nanoparticles were used by many researchers in a trial to improve the adhesive properties [4-7].
Calcium hydroxyapatite nanoparticles have received more attention in the past few years due to their biocompatibility, antimicrobial properties, and remineralizing performance [8]. Due to the high cost associated with commercial types of calcium hydroxyapatite nanoparticles, they are synthesized from seashells, fish scales, and eggshells as a natural source as well as the bones and teeth [9]. Zirconium oxide nanoparticles have high biocompatibility and wonderful esthetic and mechanical properties [10].
So, the study aims to assess the shear bond strength of modified-heliosit orthodontic adhesive with different concentrations of seashell and zirconium oxide nanoparticles. To explore the remnant of adhesive on the buccal tooth surface after brackets de-bonding. To determine any chemical reactions between the orthodontic adhesive and seashell or zirconium oxide nanoparticles.
Materials and Methods
The ethical approval with Ref. no. (UoM.Dent/ DM.L.24/ 22) for this research was obtained from the research ethical committee of the Dentistry College at Mosul University. The main materials that were used in the study were:
Nano hydroxyapatite (nHA) was manually prepared from snail seashells.
Zirconium oxide (ZrO2) Nanoparticles from (Nano shel, USA).
Heliosite orthodontic adhesive from (IVOCLARE Viva dent).
Stainless steel standard edgewise Metal brackets from (Dentarum, Germany).
Methods
Laboratory synthesis of nano-hydroxyapatite from seashell
nHA that was used in this study was manually prepared from snail seashells with a procedure illustrated by Alhussary, et al. [11] at the College of Dentistry, University of Mosul (patent 6987, A61C13/08, A61L27/12). The prepared seashell nanoparticles were further characterized by the Transmission Electron Microscope (TEM) (Philips, em208s 100Kv) at different magnification power (92000 and 130000X) to determine the shape and size of nanoparticles.
Modified orthodontic adhesive preparation
Three different concentrations of seashell-modified orthodontic adhesive (8%), (10%), and (12%) were prepared in a weight-weight ratio according to the following equation: (required nanoparticles weight = Adhesive weight × percentage of nanoparticles) [12]. the adhesive and seashell nanoparticles were accurately weighted by electrical sensitive balance (KERN, Germany). Then the two components were vigorously mixed in a semi-dark room by plastic spatula on a glass slab until homogenous form and uniform color of the adhesive material were obtained. The modified orthodontic adhesive resin was placed in a sterile disposable syringe and covered with black tape to prevent exposure to the light. The same procedure was used for the preparation of zirconium oxide-modified orthodontic adhesive at three different concentrations (1%), (3%), and (5%), but with a new glass slab.
Samples, design, and criteria of samples selection of the study
Thirty-five sound extracted premolars for orthodontic reasons were collected from governmental health centers and dental clinics in Mosul city, and were kept in distilled water [13]. Carious teeth, teeth with enamel defects, and visible cracks were excluded from the study sample collections. The design of the study is described in Figure 1.
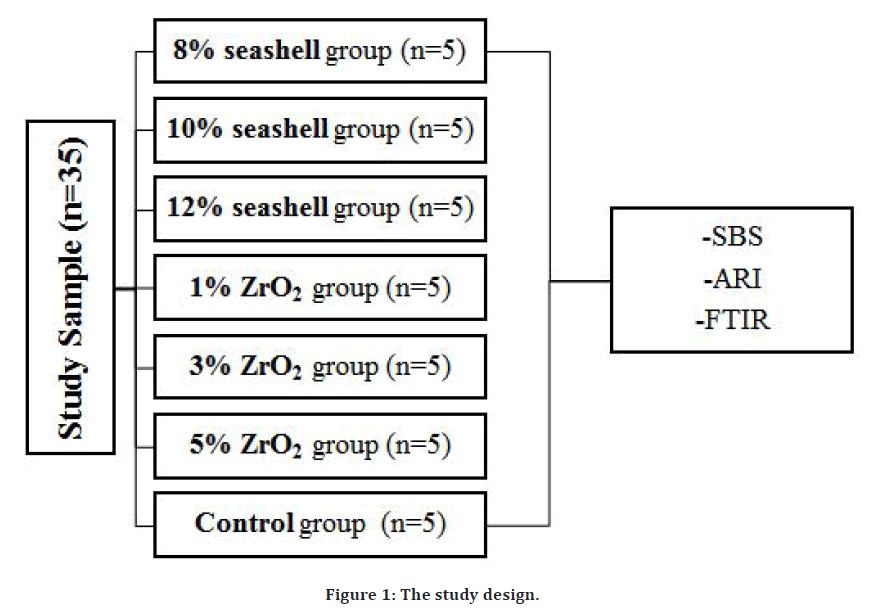
Figure 1. The study design.
Samples preparation
The collected teeth were brushed with a simple toothbrush to remove the adherent soft tissue and stored in (0.1%) thymol solution at room temperature [4]. Dental stone was poured to the half-height of a plastic (18 mm diameter) and (30 mm height) Poly Vinyl Chloride rings (PVC) until setting. Then each tooth was fixed vertically on the stone surface from the root apex by a sticky wax. Auto polymerization cold cure acrylic resin was used to fill the PVC rings to the level of the cement enamel junction so that the crown portion of the tooth emerged from the acrylic surface [14].
Bonding procedure
A low-speed handpiece (Mena, China) with rubber cups and fluoride-free pumice (PD, Germany) were used to polish all teeth samples for (10 sec.), rinsing with water, and drying the samples with airflow [14]. The buccal enamel surface of the samples was etched for (60 sec.) with phosphoric acid gel (37%) (SDI, Australia), rinsing with water for (10 sec.), drying until a chalky appearance was observed [15]. The orthodontic adhesive was distributed by a dental probe on the base of the brackets, while it was held by a clamp tweezers. To ensure correct positioning, the Bracket was placed using boons gauge on the buccal enamel surface (4-4.5 mm from the occlusal surface). We applied a known load attached to the articulator’s arm (Quick perfect, France) and directed it vertically to the bracket slots of all specimens by the anterior pin of the articulator to ensure a standard pressure and produce uniform adhesive layer thickness and to prevent air bubbles [16]. Removal of excess adhesive from the boundaries of the brackets was performed by a sharp dental explorer. Curing using a light-curing device (Benq, china) with intensity (1500 MW/cm) and (420-480 nm wavelength), at 2 mm distance from the edges of the bracket base in all samples. The total curing time was (40 sec.) for each sample, (20 sec.) at each mesial and distal side of the bracket. The specimens were placed in distilled water for 24 hr. at 37°c, [17,18]. We used a curing radiometer (china) to measure the light intensity through the polymerization of all specimens to ensure steady light intensity.
Fourier Transform Infrared Spectrometry (FTIR)
FTIR spectrophotometer device (Bruker, Germany) at the (Dentistry College, Mosul University) was used for Identification the chemical characteristics of conventional orthodontic adhesive material and after modification with seashell and zirconium oxide nanoparticles. Seven drop-like samples were prepared as a drop of heliosit orthodontic adhesive and drop of each concentration of modified orthodontic adhesives placed on a clean and sterile glass slab [15]. The samples were cured by the same time, intensity, and curing device that was used for all study samples.
Shear Bond Strength (SBS)
SBS (in MPa) is defined as the required force that remove the orthodontic bracket from a tooth surface divided by surface area of the bracket base 4. SBS was measured after 24 hours of brackets bonding at Dentistry College, Mosul University, using universal testing machine. (Gester, china). the chisel shape blade of testing machine was positioned at the interface between tooth and adhesive from occlusal-gingival direction until failure occurred. The cross-head speed was (0.5 mm/min). SBS values calculated by the following equation: “SBS in (MPa) = Force in Newton's unit / the surface area of bracket base in mm2” [12].
Adhesive Remnant Index (ARI)
The enamel surface of the buccal aspect of all specimens in the study were examined using stereomicroscope at (10X) magnification power (Optika, Italy). The criteria that were used for measuring ARI scores were: (Score 0) if there is no adhesive material remnant on the buccal enamel surface. (Score 1) if less than half of the adhesive material remained on the buccal enamel surface. (Score 2) if the remnant on the buccal surface of tooth was more than half of the adhesive material. (Score 3) means that all adhesive material was remained on the buccal surface of tooth, with a distinct mesh impression of the bracket base [19].
Results
TEM
Results of TEM examination reveal that the size of both Seashell and zirconium oxide nanoparticles ranged from (25-100 nm) and the particles were round or oval in shape as seen in Figure 2.
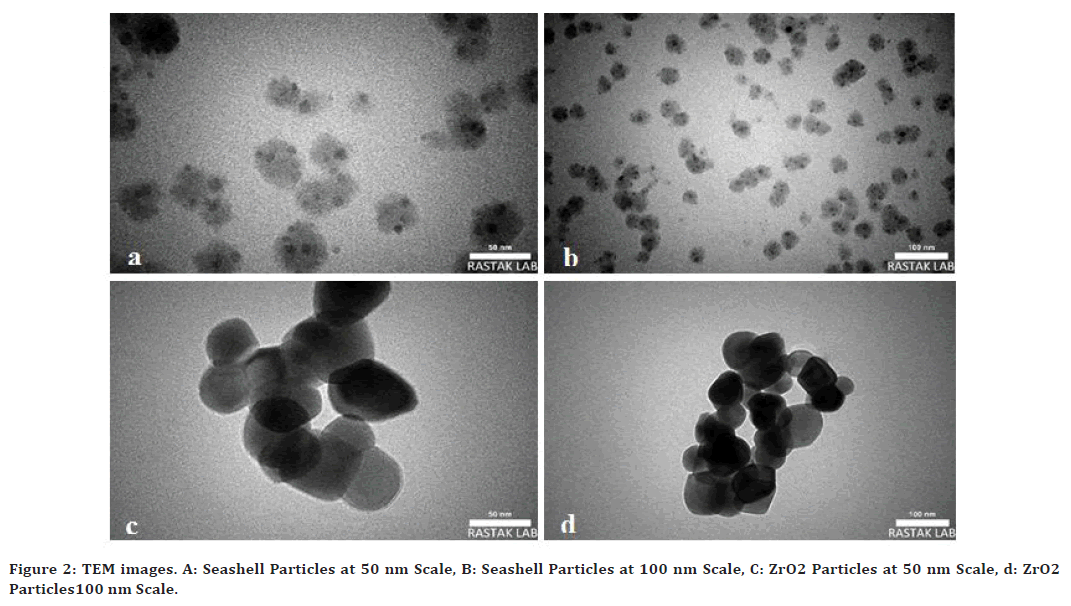
Figure 2. TEM images. A: Seashell Particles at 50 nm Scale, B: Seashell Particles at 100 nm Scale, C: ZrO2 Particles at 50 nm Scale, d: ZrO2 Particles100 nm Scale.
FTIR
Figure 3 illustrate the appearance of some bands at 2956, 1717, and 1296 wave- number-cm-1, by FTIR spectra of control group which are due to C-H stretching and C-O., the same bands at the same region appeared by FTIR spectra of the modified orthodontic adhesive, which showed no shifting or disappearing bands when compared with control group spectra as seen in.
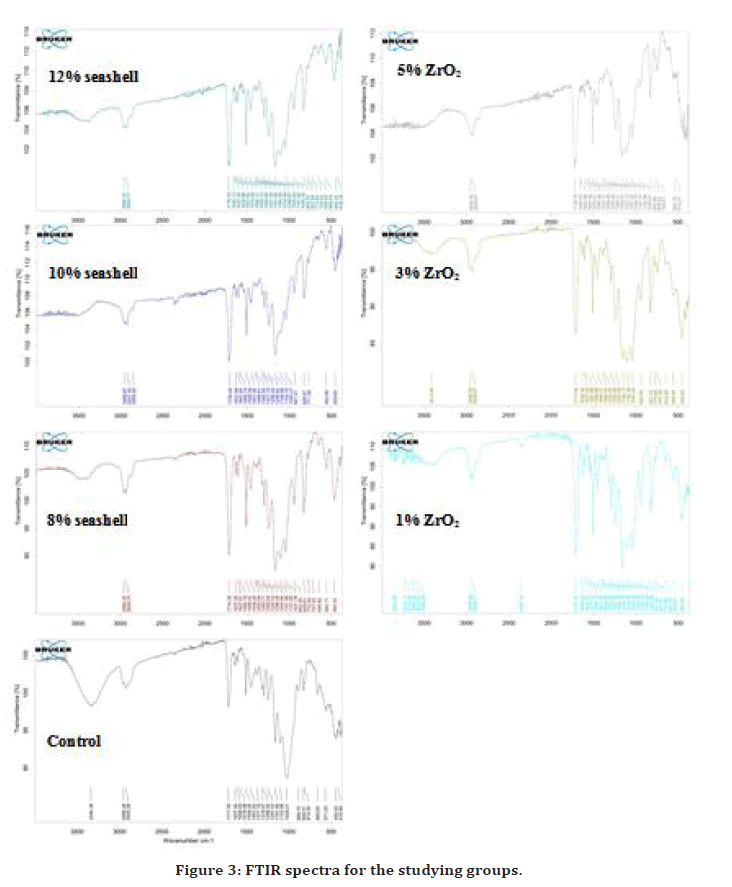
Figure 3. FTIR spectra for the studying groups.
SBS
Table 1 show the descriptive statistics including the sample number per group, mean ± standard deviation, minimum, and maximum values, in addition to Duncun Multiple Range test. According to these results, the 10% seashell group had the highest mean value followed 8% seashell group, while the 5% ZrO2 group showed the lowest SBS mean values. The data analysis by oneway ANOVA and post hoc Duncan's Multiple Range tests showed that there were no significant differences among control and other studying groups except between 5% ZrO2 group and 8%, 10% seashell groups.
| Groups | N | Min | Max | Mean ± SD |
|---|---|---|---|---|
| Control | 5 | 5.99 | 11.62 | 8.97 ± 2.36 ab |
| 8% seashell | 5 | 8.63 | 13.78 | 11.17 ± 1.92 b |
| 10% seashell | 5 | 9.53 | 13.64 | 11.19 ± 1.79 b |
| 12% seashell | 5 | 6.5 | 13.1 | 9.61 ± 2.50 ab |
| 1% ZrO2 | 5 | 6.04 | 10.32 | 8.49 ± 1.91 ab |
| 3% ZrO2 | 5 | 3.23 | 14.13 | 8.18 ± 4.45 ab |
| 5% ZrO2 | 5 | 6.72 | 7.17 | 6.93 ± 0.18 a |
| Sig. | 0.094 | |||
Table 1: Descriptive statistics of shear bond strength.
ARI
Table 2 represent the descriptive statistics of ARI including mean, minimum, maximum score, standard deviation for each group in the study, and significant value of the Kruskal-Wallis test. Analysis of data subjected that the least mean ARI scores were in 10% seashell and 1% ZrO2 groups, while the highest mean ARI scores were in 8% seashell and 5% ZrO2 groups. The percentages of mean score for each group in the study are described in Figure 4 showing the 10% seashell group has the least percentage of mean score. However, no significant differences were presented by Kruskal-Wallis test among the mean scores of the groups compared to the control group.
| Groups | N | Min | Max | Mean ± SD |
|---|---|---|---|---|
| Control | 5 | 1 | 3 | 1.8 ± 0.84 |
| 8% seashell | 5 | 2 | 3 | 2.2 ± 0.45 |
| 10% seashell | 5 | 1 | 3 | 1.6 ± 0.89 |
| 12% seashell | 5 | 1 | 3 | 1.8 ± 0.84 |
| 1%ZrO2 | 5 | 1 | 3 | 1.6 ± 0.89 |
| 3% ZrO2 | 5 | 1 | 2 | 1.8 ± 0.45 |
| 5%ZrO2 | 5 | 2 | 3 | 2.2 ± 0.45 |
| Sig. | 0.606 | |||
Table 2: Descriptive statistics of ARI.
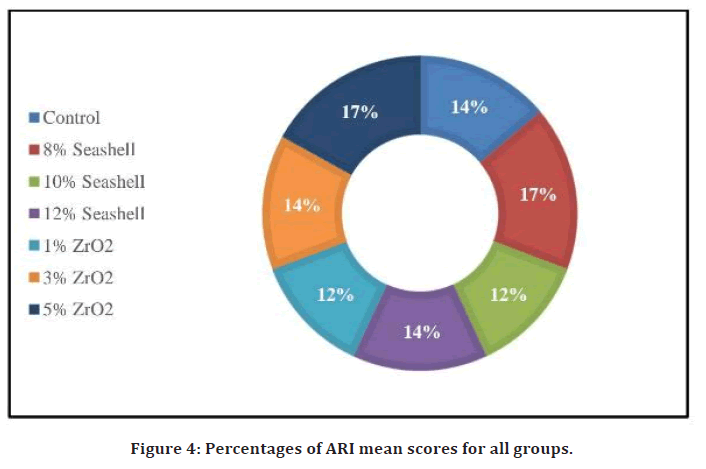
Figure 4. Percentages of ARI mean scores for all groups.
Discussions
This study employed a possible solution to enhance the bonding strength of orthodontic adhesive and prevent debonding of orthodontic brackets, which was the addition of seashell and ZrO2 nano-filler to the orthodontic adhesive. nHA contains calcium and phosphate remineralizing ions and is highly biocompatible [20]. These advantages encouraged us to incorporate nHA derived from natural seashells in the adhesive due to affordable production and biocompatibility and analyze their particle’s shape and size by transmission electron microscope, measure SBS to represent the experimental groups ability to resist de-bonding force, and identify a possible chemical reaction between nanoparticles and adhesive by FTIR.
Observation of seashell and ZrO2 nanoparticles under TEM revealed that the particle’s sizes were ranging from (25-100) nm with round or ovoid shape, the spherical shaped particles have a lubricating effect on the material so increase its flow ability, whereas amour phase particles cause an increase in its viscosity according to Aljamhan, et al. [20].
According to the results of this study, (8, 10, 12 %) seashell groups have higher mean SBS values than the control group, so the addition of (8, 10, 12 %) seashell containing nHA to Heliosit adhesive have a better performance on SBS of orthodontic adhesive even though the difference was not significant from control group. This may be due to the presence of Nano filler that absorb more and withstand the applied stress on the brackets because it acts as an elastic layer according to Akhavan, et al., and Hasan, et al. [4,12]. Higher SBS mean value may be associated with the remineralization of tooth surface from hydroxyapatite nanoparticles according to Scribante, et al. and Enan, et al. [21,22]. This result agreed with Elsharkawy, et al. [6], who conclude that (20%) added Nano filler yielded lesser strength than (10%), but disagree with Tabaii, et al. [23], who conclude that 10% nHA “reduced shear bond strength of resin reinforced glass ionomer cement modified by nHA on ceramic bracket de-bonding”, this difference may be due to different adhesive material. Reduced SBS mean value of (12%) seashell group lesser than (8 and 10%) seashell groups this may be attributed to adding more fillers that may increase adhesive viscosity, agglomeration of nanoparticles, and increased porosities within the orthodontic resin matrix that leading to water absorption and incomplete penetration of calcium ions [8].
In this study, orthodontic adhesive modified with ZrO2 nanoparticles in (1, 3, and5%) groups neither improved nor compromised the mean SBS values compared to control group as their mean SBS values remain near the values of control group with no significant differences. However, all studying groups were showed higher mean SBS values than the clinically acceptable range (5.9 to 7.8 MPa) that suggested by Reynolds, et al. [24], except 5% ZrO2 nanoparticles group is within clinically adequate SBS. The results of ZrO2 groups disagree with Al-Saleh, et al., [10], they concluded that reinforcing the experimental adhesive with ZrO2 nanoparticles has a higher mean value than control group, this may be due to the difference in methodology working on dentin, and using thermo cycling and non-thermo cycling protocol.
To explore the remnant of orthodontic adhesive material on the buccal enamel surfaces after brackets debonding, ARI was performed. There was no significant difference among all study groups that’s mean the addition of different concentrations of (1,3,5%) ZrO2 and (8,10,12%) seashell nanoparticle didn’t increase the amount of adhesive remained on tooth surface after de-bonding. Low ARI scores may be beneficial because it leads to easy orthodontic bracket removal, less iatrogenic damage to the tooth by the orthodontist, and easy rebonding procedure during orthodontic treatment [25].
The FTIR spectra for the control and modified adhesive groups with (1,3,5%) ZrO2 and (8.10.12%) seashell nanoparticles, showed the same bands and positions without shifting or changing. That’s means the modification of heliosit orthodontic adhesive with seashell and ZrO2 nanoparticle were not revealed any chemical reactions or changes or formation of a new material.
Conclusion
Addition of (8, 10, 12 %) Seashell containing nano hydroxyapatite had better performance and improved shear bond strength, while (1, 3, 5 %) zirconium oxide nanoparticles did not compromise the shear bond strength of heliosit orthodontic adhesive. Addition of seashell and zirconium oxide nanoparticles did not violate the amount of adhesive that remained on buccal enamel surfaces after de-bonding of brackets, and did not induce any chemical reaction with heliosit orthodontic adhesive.
Consent to Participate
We, the authors, guarantee that we participated in the completion of the manuscript. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
We, the authors agree to publish the manuscript in your journal.
Competing Interests
There are no competing interests for this work.
Funding
There is no funding.
Authors' Contributions
The practical intervention related to 1st author, the idea sharing for both authors, arch measurement for the 2nd author, the writing of the research for both authors.
Acknowledgements
To my college of dentistry, University of Mosul.
References
- Arhun N, Arman A, Cehreli SB, et al. Microleakage beneath ceramic and metal brackets bonded with a conventional and an antibacterial adhesive system. Angle Orthod 2006; 76:1028-1034.
- Sodagar A, Akhoundi MS, Bahador A, et al. Effect of TiO 2 nanoparticles incorporation on antibacterial properties and shear bond strength of dental composite used in Orthodontics. Dent Press J Orthod 2017; 22:67-74.
- Felemban NH, Ebrahim MI. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: An in vitro study. BMC Oral Health 2017; 17:1-8.
- Akhavan A, Sodagar A, Mojtahedzadeh F, et al. Investigating the effect of incorporating nanosilver/nanohydroxyapatite particles on the shear bond strength of orthodontic adhesives. Acta Odontol Scandinavica 2013; 71:1038-1042.
- Altmann AS, Collares FM, Balbinot GD, et al. Niobium pentoxide phosphate invert glass as a mineralizing agent in an experimental orthodontic adhesive. Angle Orthod 2017; 87:759-765.
- DA E. Effect of incorporation of bioactive glass nano particles in adhesive resin on microtensile bond strength to dentin in self etch and etch and rinse mode. Al-Azhar Dent J Girls 2018; 5:399-404.
- Callister C, Callister M, Nolan M, et al. Remineralization potential of a novel silver nanoparticle rinse on severely demineralized enamel In-vitro: A 14-day trial. J Nanomed Nanotechnol 2020; 11:1-5.
- Nobre CM, Pütz N, Hannig M. Adhesion of hydroxyapatite nanoparticles to dental materials under oral conditions. Scanning 2020; 2020.
- Majhooll AA, Zainol I, Jaafar CN, et al. A brief review on biomedical applications of hydroxyapatite use as fillers in polymer. J Chem 2019; 13:112-119.
- Al-Saleh S, Alateeq A, Alshaya AH, et al. Influence of TiO2 and ZrO2 nanoparticles on adhesive bond strength and viscosity of dentin polymer: A physical and chemical evaluation. Polymers 2021; 13:3794.
- Alhussary BN, A Taqa G, Taqa AA. Preparation and characterization of natural nano hydroxyapatite from eggshell and seashell and its effect on bone healing. J Appl Vet Sci 2020; 5:25-32.
- Hasan LA. Evaluation the properties of orthodontic adhesive incorporated with nano-hydroxyapatite particles. Saudi Dent J 2021; 33:1190-1196.
- Cerone M, El-Badrawy W, Gong SG, et al. Bond strength of universal self-etch 1-step adhesive systems for orthodontic brackets. J Can Dent Assoc 2019; 85:6.
- Rodrigues-Tonetto M, Alves de Campos E, Fernández E, et al. Bond strength and adhesive remnant index of experimental brackets bonded with self-adhesive resin cement. Revista Clín Period Implantol Rehab Oral 2017; 10:115-117.
- Al Khayat ZE, Al Hamdany AKS. Shear and tensile bond strengths of titanium dioxide nanoparticles modified orthodontic adhesive. Int J Enhanced Res Med Dent Care 2018; 5:1-10.
- Degrazia FW, Genari B, Leitune VC, et al. Polymerisation, antibacterial and bioactivity properties of experimental orthodontic adhesives containing triclosan-loaded halloysite nanotubes. J Dent 2018; 69:77-82.
- Blöcher S, Frankenberger R, Hellak A, et al. Effect on enamel shear bond strength of adding microsilver and nanosilver particles to the primer of an orthodontic adhesive. BMC Oral Health 2015; 15:1-9.
- Vargas EO, Nuernberg CC, Maciel JV, et al. Influence of primekote® polymer in orthodontic bonding. Rev Odontol UNESP 2017; 46:61-65.
- Yaseen SN, Taqa AA, Al-Khatib AR. The effect of incorporation nano cinnamon powder on the shear bond of the orthodontic composite (an in vitro study). J Oral Biol Craniofac Res 2020; 10:128-134.
- Aljamhan AS, Alrefeai MH, Alhabdan A, et al. Influence of ER-CR-YSGG laser and photodynamic therapy on the dentin bond integrity of nano-hydroxyapatite containing resin dentin adhesive: SEM-EDX, micro-raman, micro-tensile, and FTIR evaluation. Polymers 2021; 13:1903.
- Scribante A, Dermenaki Farahani MR, Marino G, et al. Biomimetic effect of nano-hydroxyapatite in demineralized enamel before orthodontic bonding of brackets and attachments: Visual, adhesion strength, and hardness in in vitro tests. BioMed Res Int 2020; 2020.
- Enan E, Tawfik MA, Mehesen R, et al. Remineralization potential and shear bond strength of surface treated hypomineralized enamel in bonding of orthodontic brackets: An in vitro study. J Adv Oral Res 2021; 12:127-133.
- Tabaii ES, Sari MN. Evaluation of shear bond strength of resin reinforced glass ionomer cement modified by nano-hydroxyapatite on ceramic bracket debonding using full-dimension wire. Annual Res Rev Biol 2014; 1578-1586.
- Reynolds IR. A review of direct orthodontic bonding. Br J Orthod 1975; 2:171-178.
- Kechagia A, Zinelis S, Pandis N, et al. The effect of orthodontic adhesive and bracket-base design in adhesive remnant index on enamel. J World Fed Orthod 2015; 4:18-22.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Alaa Gharis Adnan1 and Neam Agha2*
1Ministry of Health, Nineveh Health Directorate, Mosul, Iraq2Department of Pedodontic, Orthodontic, and Preventive Dentistry, College of Dentistry, University of Mosul, Iraq
Received: 03-Sep-2022, Manuscript No. jrmds-22-74108; , Pre QC No. jrmds-22-74108(PQ); Editor assigned: 05-Sep-2022, Pre QC No. jrmds-22-74108(PQ); Reviewed: 19-Sep-2022, QC No. jrmds-22-74108(Q); Revised: 23-Sep-2022, Manuscript No. jrmds-22-74108(R); Published: 30-Sep-2022
