Research - (2021) Volume 9, Issue 6
Morphological Study of Endometrial Blood Vessels
Firdaus Ali and Hemalatha Ganapathy*
*Correspondence: Hemalatha Ganapathy, Department of Pathology, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, India, Email:
Abstract
Endometrial blood vessels are under the influence of hormones. Hence, a comparative study of average number of blood vessels per 10 HPF between hormonally normal endometrium and pathological conditions can yield meaningful results in understanding the vascularity of endometrium. The present study was designed to evaluate the number of blood vessels per 10 HPF in the endometrium of specimens of patients. In this regard, uterine endometrial specimens were obtained by hysterectomy and D&C and patients were divided in two groups - control and study (DUB, endometriosis, adenomyosis and leiomyoma). The average number of blood vessels per 10 HPF were concentrated more in the basalis layer during proliferative phase and more in the functionalism layer during secretory phase. The average number of blood vessels per 10 HPF in endometriosis was significantly higher (5.50 ± 0.70) and found in leiomyoma and endometriosis when compared with the control. In conclusion a positive correlation between endometrial angiogenesis and uterine pathologies is observed.
Keywords
DUB, Endometriosis, Adenomyosis, Leiomyoma, Angiogenesis, Uterine pathology
Introduction
Endometrial vasculature is unique because it undergoes functional and cyclical changes during every menstrual period in the reproductive age group. The number and morphology of blood vessels vary in different conditions, both normal and pathological. Study of blood vessels has been extensively undertaken especially in malignant conditions, to evaluate neo angiogenesis. The demonstration of neo angiogenesis involves sophisticated techniques like immunohistochemistry and ultrastructural studies which cannot be applied in routine cases. This study aims at evaluation of endometrial blood vessels using the routine processing and staining techniques and to correlate with normal and uterine pathological conditions. Since endometrial blood vessels are under the influence of hormones, a comparative study of average number of blood vessels per 10 HPF between hormonally normal endometrium and pathological conditions can yield meaningful results in understanding the vascularity of endometrium.
After fertilization endometrium undergoes precisely defined morphological changes until a receptive endometrium is developed. These morphological changes were described as early as 1950 by Noyes, Hertig and Rock and these changes occur under the control of the sexual steroid hormones, estrogen and progesterone. Estrogen is the dominant hormone of the proliferative phase [1], whereas progesterone is the determining hormone of the secretory phase, where transformation and stromal decidualization occur [2]. Spiral arterioles are the important arteries which supply the endometrium and myometrium. These spiral arteries are more coiled in the secretory phase of menstrual cycle [3]. The basal portions of the blood vessels are unchanged throughout the menstrual cycles, but the functional portion of the endometrium is constantly changing.
Endothelial cell proliferation occurs during angiogenesis. It can be demonstrated by immunohistochemistry to evaluate the endothelial cell proliferation in different endometrial diseases and different phases of endometrium. Neo angiogenesis is identified by new vascular growth in endometrium. Due to this vascular growth, variation of angiogenesis occurs in different phases and diseases of endometrium. Neovascularisation also occurs during implantation of blastocysts. Increased vascularisation also indicates embryo implantation. The number of blood vessels and the endometrial blood flow increases during the proliferative phase.
During ovulatory menstrual cycles in early reproductive life, there is an angiogenetic effect which permits successful implantation and placentation. Increased neovascularisation may also lead to the development of uterine pathologies. Defects in angiogenesis in female reproductive system may play a role in the development of pathological disorders. A comparative study of average number of blood vessels per 10 HPF between hormonally normal endometrium and pathological conditions can yield meaningful results in understanding the vascularity of endometrium. Hence, the present study was designed to evaluate the number of blood vessels per 10 HPF in the endometrium of specimens of patients.
Materials and Methods
Method
The eighty five specimens obtained were of DUB, local uterine lesions like leiomyoma, adenomyosis and endometriosis. Cases of infertility and atrophic uterus were not included in the focus of this study. The number of study cases taken were DUB -33, Adenomyosis -10, Endometriosis -6, Leiomyoma -36.
Clinical data were obtained from cases registered and pertinent patient details like name, age, sex, hospital registration number, clinical diagnosis were included in this study.
Required sections were taken from Hysterectomy and Dilatation and Curettage (D&C) specimens. The tissues were fixed in 10% formalin and proceeded routinely. Sections of 4–5-micron thickness were stained with Hematoxylin and Eosin (H&E) stain.
Procedure
• Dewaxing done with xylin 2 changes, each for 10 minutes,
• Then the slide was put in alcohol 2 changes, each for one minute duration.
• Then the slide was washed with water.
• Hematoxylin (Mayer's) put for 15 minutes.
• Slide was again washed with water.
• Dipped in acid alcohol twice.
• The slide was washed with water for 2 minutes 20 Seconds.
• Lithium carbonate was poured over the slide until blueing occurred.
• Then the slide was again washed with water for one minute.
• Eosin was poured on the slide for 20 seconds.
• The slide was washed with water again.
• The slide was kept in air to dry.
• Slide was mounted with DPX (Disteme Dibutyl Phthalate Xylene).
Results: Blue coloured nucleus; Pink coloured cytoplasm.
In cases of leiomyoma, Van Gieson staining was done. Van Gieson' s solution contains acid fuchsin 1% (5ml) and picric acid saturated solution (95ml).
Procedure
• The thickness of 4-5 microns sections are deparaffinised and hydrated with distilled water.
• Sections were stained with Hematoxylin for 15 minutes.
• The slide was then washed under running tap water for 10 minutes.
• The sections were then stained with Van Gieson stain for 3 minutes.
• The slide was then put in 95% Alcohol.
• The slide was then dehydrated and mounted with DPX.
Results: Black coloured nucleus; Brilliant red coloured collagen and Yellow coloured muscles and cytoplasm.
The overall vascularity of the endometrium was judged by counting the average number of blood vessels seen per 10 High Power Field (HPF) in the basal layer and functional layers separately and compared with the control.
Criteria for control group
Hysterectomy specimens performed for hormonally normal endometrium presented as prolapse uterus were taken as control in the age group 35-65 years (Table 1).
| Age group | Number of control cases |
|---|---|
| 35-45 Years | 4 |
| 46-55 Years | 5 |
| 56-65 Years | 1 |
Table 1: Hysterectomy.
Endometrial curettage performed along with cervical biopsy with no suppression of endometrial pathology with the age group 35-65 years (Table 2).
| Age group | Number of control cases |
|---|---|
| 35-45 Years | 3 |
| 46-55 Years | 2 |
| 56-65 Years | 0 |
Table 2: Endometrial curettage.
Statistical indices
In the present study, the average (mean) and standard deviation values have been calculated.
Average
Average=Sum of all the values/total number of values.
Standard deviation
Standard deviation is a widely used measure of variability or diversity used in statistics and probability theory denoted by (±). It shows how much variation or dispersion exists from the average (mean or expected value). A low standard deviation indicates that the data points tend to be awfully close to their mean, whereas high standard deviation indicates that the data points are spread over a large range of values.
Results
In the control group, the number of cases taken were fifteen in which 8 were in early proliferative, 4 were in late proliferative, 3 were in early secretory and nil cases in late secretory phase in hysterectomy and D&C specimens (Table 3). There was statistically significant difference in the endometrial blood vessel density in different phases of endometrium in stratum basalis and stratum functionalis. The blood vessels are mainly concentrated in the basal layer in the proliferative phase and were concentrated more in the functional layer in secretory phase. The study group included 85 endometrial specimens. Thirty-six cases were of leiomyoma, six cases were of endometriosis, ten cases of adenomyosis and thirty three cases of DUB (Table 4).
| S. No | Histopathological pattern | No. of Cases |
|---|---|---|
| 1 | EPP | 8 |
| 2 | LPP | 4 |
| 3 | ESP | 3 |
| 4 | LSP | 0 |
Table 3: Control group.
| S. No | Histopathological pattern | No. of blood vessels in basalis | No. of blood vessels in functionalism |
|---|---|---|---|
| 1 | EPP | 4.0 ± 1.51 | 3.12 ± 1.24 |
| 2 | LPP | 2.62 ± 2.10 | 1.87 ± 1.55 |
| 3 | ESP | 1.12 ± 1.55 | 1.25 ± 1.64 |
| 4 | LSP | 0 | 0 |
Table 4: Average number of blood vessels per 10 HPF in the control.
In cases of DUB, 6 cases were in early proliferative, 9 were in late secretory, 15 were in early secretory and 3 were in late secretory phase respectively (Table 5). There was statistically significant difference observed in the endometrial blood vessel density in different phases of endometrium in stratum basalis and stratumfunctionalis. The blood vessels are mainly concentrated in the basalis layer in both proliferative and secretory phase (Table 6).
| S. No | Histopathological pattern | No. of cases |
|---|---|---|
| 1 | EPP | 6 |
| 2 | LPP | 9 |
| 3 | ESP | 15 |
| 4 | LSP | 3 |
Table 5: Number of cases of DUB in different phases of endometrium.
| S. No | Histopathological pattern | No. of blood vessels basalis | No. of blood vessels in functionalis |
|---|---|---|---|
| 1 | EPP | 2.06 ± 2.65 | 1.20 ± 1.56 |
| 2 | LPP | 2.33 ± 1.99 | 1.86 ± 1.72 |
| 3 | ESP | 3.13 ± 1.12 | 2.93 ± 1.03 |
| 4 | LSP | 0.60 ± 1.24 | 0.46 ± 0.99 |
Table 6: Average number of blood vessels per 10 HPF in DUB cases.
There was statistically significant difference seen in the endometrial blood vessel density in different phases of endometrium in stratum basalis and stratum functionalis. The blood vessels are seen to be more concentrated in the basalis layer in the proliferative phase in cases of adenomyosis (Table 7). Statistically significant difference is observed in the endometrial blood vessel density in different phases of endometrium in stratum basalis and stratum functionalis. The blood vessels are seen to be more concentrated in the basalis layer in the proliferative phase in cases of endometriosis (Table 8).
| HP Pattern | Average blood vessels/HPF | |
|---|---|---|
| Basalis | Funtionalis | |
| EPP | 4.0 ± 0.81 | 3.25 ± 0.95 |
| LPP | 2.03 ± 2.30 | 1.50 ± 1.73 |
| ESP | 2.25 ± 1.70 | 2.75 ± 1.89 |
| LSP | 0.75 ± 1.50 | 1.00 ± 2.50 |
Table 7: Average number of blood vessels per 10 HPF in adenomyosis.
| HP Pattern | Average blood vessels/HPF | |
|---|---|---|
| Basalis | Funtionalis | |
| EPP | 5.50 ± 0.70 | 3.50 ± 0.70 |
| LPP | 2.0 ± 2.82 | 1.50 ± 2.12 |
| ESP | 3.0 ± 0.00 | 3.50 ± 0.70 |
| LSP | 0.91 ± 1.37 | 0 |
Table 8: Average number of blood vessels per 10 HPF in endometriosis.
Statistically significant difference was seen in the endometrial blood vessel density in different phases of endometrium in stratum basalis and stratum functionalis. The blood vessels are more concentrated in the basalis layer in the proliferative phase in cases of fibroid. In DUB, based on histopathological diagnosis, the average number of blood vessels per 10 HPF in early proliferative, late proliferative, early secretory, late secretory phases was compared with the control (Table 9). The average blood vessels per 10 HPF were significantly higher m control (4.0 ± 1.51) as compared to DUB (0.46 ± 0.99). There was statistically significant difference in the endometrial blood vessel density in different phases of endometrium in stratum basalis and stratum functionalis. The blood vessels are mainly concentrated more in the basalis layer in the proliferative phase in the control group and were more concentrated in the basalis layer in the secretory phase in the DUB cases (Table 10).
| HP Pattern | Average blood vessels/HPF | |
|---|---|---|
| Basalis | Funtionalis | |
| EPP | 4.50 ± 1.00 | 3.33 ± 0.65 |
| LPP | 3.66 ± 1.23 | 3.83 ± 0.71 |
| ESP | 2.16 ± 1.33 | 2.83 ± 1.85 |
| LSP | 0.91 ± 1.37 | 1.41 ± 1.83 |
Table 9: Average number of blood vessels per 10 HPF in leiomyoma.
| HP Pattern | Control | DUB | ||
|---|---|---|---|---|
| Basalis | Funtionalis | Basalis | Funtionalis | |
| EPP | 4.0 ± 1.51 | 3.12 ± 1.24 | 2.06 ± 2.65 | 1.20 ± 1.56 |
| LPP | 2.62 ± 2.19 | 1.87 ± 1.55 | 2.33 ± 1.99 | 1.86 ± 1.72 |
| ESP | 1.12 ± 1.55 | 1.25 ± 1.64 | 3.13 ± 1.12 | 2.93 ± 1.03 |
| LSP | 0 | 0 | 0.60 ± 1.64 | 0.46 ± 0.99 |
Table 10: Comparison of average endometrial blood vessels of control with DUB.
This is maybe, since, the increased density of blood vessels combined with the fragile nature of these vessels maybe responsible for the bleeding in DUB. Therefore, it is possible that bleeding in DUB maybe related to some qualitative changes in the blood vessels. It is also possible that the various morphological changes in the endometrial vasculature in DUB and rupture of dilated and congested vascular channels could be responsible for the dysfunctional uterine bleeding. In Adenomyosis, ten cases were included in the study. All were hysterectomy specimens. On basis of histopathological diagnosis, the average number of blood vessels per 10 HPF in early proliferative, late proliferative, early secretory, late secretory phases was compared with the control in Table 11. There was no statistically significant difference in blood vessel density in adenomyosis and control group.
| HP Pattern | Control | DUB | ||
|---|---|---|---|---|
| Basalis | Funtionalis | Basalis | Funtionalis | |
| EPP | 4.0 ± 1.51 | 3.12 ± 1.24 | 4.0 ± 0.81 | 3.25 ± 0.95 |
| LPP | 2.62 ± 2.19 | 1.87 ± 1.55 | 2.03 ± 2.30 | 1.50 ± 1.73 |
| ESP | 1.12 ± 1.55 | 1.25 ± 1.64 | 2.25 ± 1.70 | 2.75 ± 1.89 |
| LSP | 0 | 0 | 0.75 ± 1.50 | 1.00 ± 2.50 |
Table 11: Comparison of average endometrial blood vessels of control with adenomyosis.
The control group has been compared to Endometriosis cases on basis of average blood vessels per HPF in different phases of endometrium.
The average number of blood vessels per 10 HPF was significantly higher in endometriosis (5.50 ± 0.70) as compared to control (1.12 ± 1.55). There was statistically significant difference in the endometrial blood vessel density in different phases of endometrium in basalis and functionalis layers. The blood vessels are more concentrated in the basalis layer in the proliferative phase in the endometriosis cases (Table 12). The average number of blood vessels per 10 HPF is more in leiomyoma (4.50 ± 1.00) when compared to control (1.12 ± 1.55). A statistically significant difference is seen in endometrial blood vessel density in basalis and functionalis layers. The blood vessels are more concentrated in the basalis layer in the proliferative phase and more in the functionalis layer in the secretory phase (Table 13).
| HP Pattern | Control | DUB | ||
|---|---|---|---|---|
| Basalis | Funtionalis | Basalis | Funtionalis | |
| EPP | 4.0 ± 1.51 | 3.12 ± 1.24 | 5.50 ± 0.70 | 3.50 ± 0.70 |
| LPP | 2.62 ± 2.19 | 1.87 ± 1.55 | 2.00 ± 2.82 | 1.50 ± 2.12 |
| ESP | 1.12 ± 1.55 | 1.25 ± 1.64 | 3.0 ± 0.00 | 3.50 ± 0.70 |
| LSP | 0 | 0 | 0.91 ± 1.37 | 0 |
Table 12: Comparison of average endometrial blood vessels of control with endometriosis.
| HP Pattern | Control | DUB | ||
|---|---|---|---|---|
| Basalis | Funtionalis | Basalis | Funtionalis | |
| EPP | 4.0 ± 1.51 | 3.12 ± 1.24 | 4.50 ± 1.00 | 3.33 ± 0.65 |
| LPP | 2.62 ± 2.19 | 1.87 ± 1.55 | 3.66 ± 1.23 | 3.83 ± 0.71 |
| ESP | 1.12 ± 1.55 | 1.25 ± 1.64 | 2.16 ± 1.33 | 2.83 ± 1.85 |
| LSP | 0 | 0 | 0.91 ± 1.37 | 1.41 ± 1.83 |
Table 13: Comparison of average endometrial blood vessels of control with leiomyoma.
Based on histopathological pattern, the average number of blood vessels per HPF in the study cases i.e., DUB, Adenomyosis, Endometriosis and leiomyoma cases have been compared with each other.
The average number of blood vessels per 10 HPF was significantly higher in endometriosis (5.50 ± 0.70) as compared to other study and control cases.
Based on histopathological pattern, in early proliferative phase, the average blood vessels were significantly higher in endometriosis (5.50 ± 0.70) in early proliferative phase and least was seen in DUB (2.06 ± 2.65). In early secretory phase, leiomyoma shows maximum vessel density. No statistically significant difference is seen in secretory phase of endometrium in the study cases (Table 14). Results are further explained in the form of figures (Figure 1 to Figure 11).
| Average blood vessels per 10 HPF | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HP Pattern | Control | DUB | Adenomyosis | Endometriosis | Leiomyoma | |||||
| Basalis | Funtionalis | Basalis | Funtionalis | Basalis | Funtionalis | Basalis | Funtionalis | Basalis | Funtionalis | |
| EPP | 4.0 ± 1.51 | 3.12 ± 1.24 | 2.06 ± 2.65 | 1.20 ± 1.56 | 4.0 ± 0.81 | 3.25 ± 0.95 | 5.50 ± 0.70 | 3.50 ± 0.70 | 4.50 ± 1.00 | 3.33 ± 0.65 |
| LPP | 2.62 ± 2.19 | 1.87 ± 1.55 | 2.33 ± 1.99 | 1.86 ± 1.72 | 2.03 ± 2.30 | 1.0 ± 1.73 | 2.0 ± 2.82 | 1.50 ± 2.12 | 3.66 ± 1.23 | 3.83 ± 0.71 |
| ESP | 1.12 ± 1.55 | 1.25 ± 1.64 | 3.13 ± 1.12 | 2.93 ± 1.03 | 2.25 ± 1.70 | 2.75 ± 1.89 | 3.0 ± 0.00 | 3.50 ± 0.70 | 2.16 ± 1.33 | 2.83 ± 1.85 |
| LSP | 0 | 0 | 0.60 ± 1.24 | 0.46 ± 0.99 | 0.75 ± 1.50 | 1.00 ± 2.50 | 0.91 ± 1.37 | 0 | 0.91 ± 1.37 | 1.41 ± 1.83 |
Table 14: Comparison of average blood vessels per 10 HPF in the study cases.
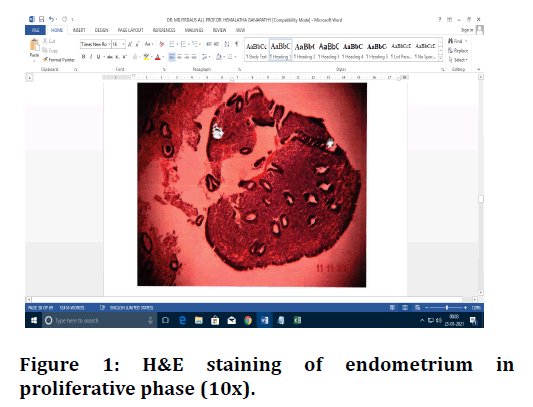
Figure 1. H&E staining of endometrium in proliferative phase (10x).
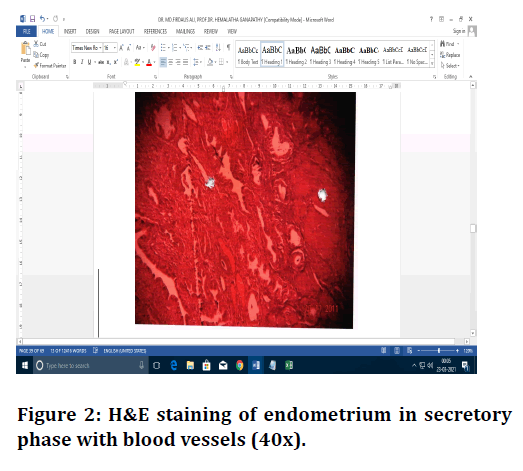
Figure 2. H&E staining of endometrium in secretory phase with blood vessels (40x).
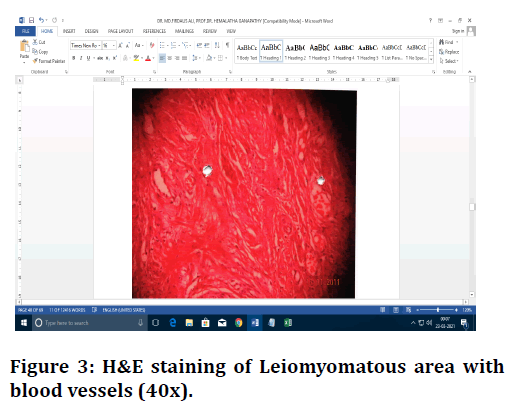
Figure 3. H&E staining of Leiomyomatous area with blood vessels (40x).
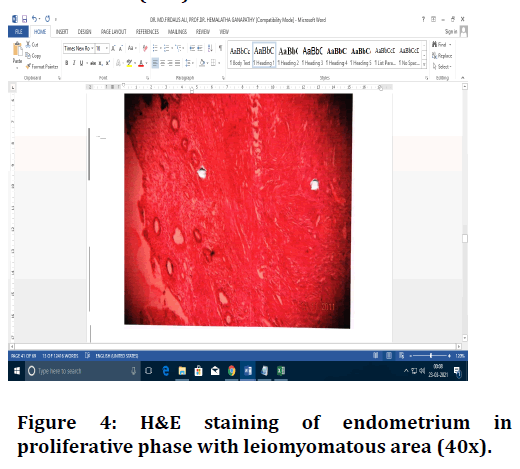
Figure 4. H&E staining of endometrium in proliferative phase with leiomyomatous area (40x).
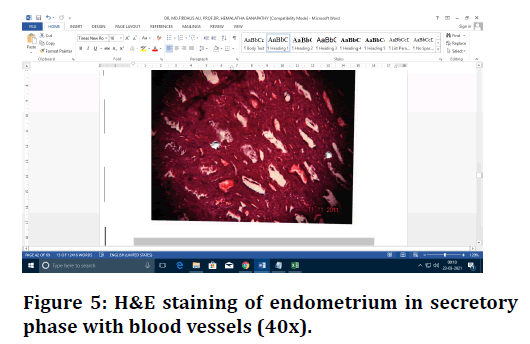
Figure 5. H&E staining of endometrium in secretory phase with blood vessels (40x).
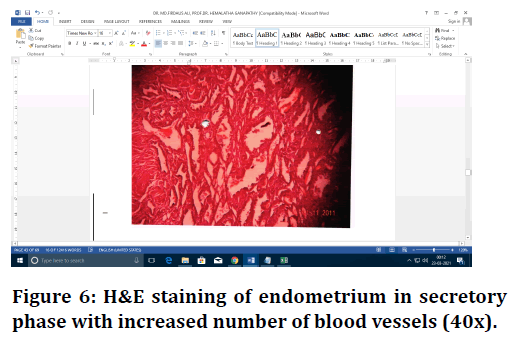
Figure 6. H&E staining of endometrium in secretory phase with increased number of blood vessels (40x).
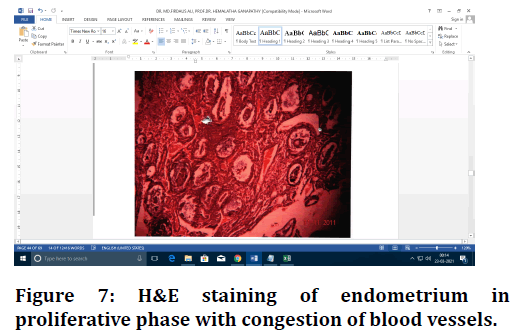
Figure 7. H&E staining of endometrium in proliferative phase with congestion of blood vessels.
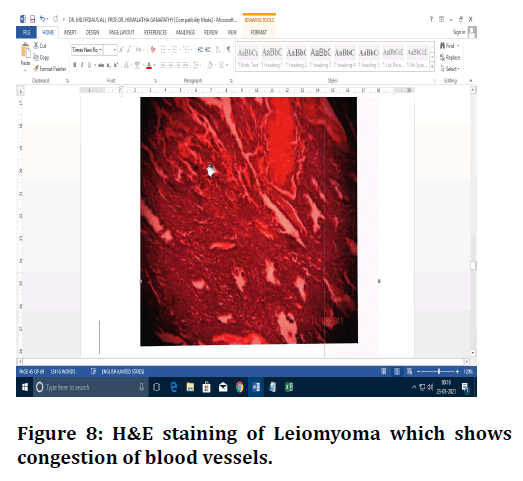
Figure 8. H&E staining of Leiomyoma which shows congestion of blood vessels.

Figure 9. Adenomyosis- H&E staining of endometrium in p oliferative phase with blood vessels (10x).
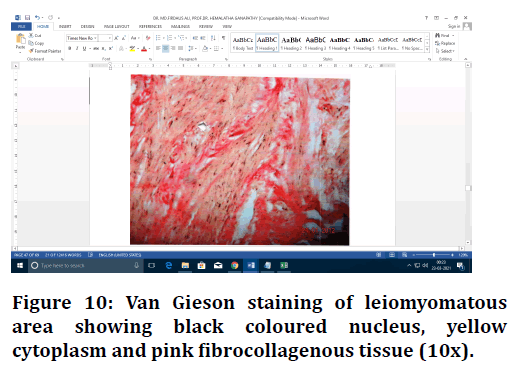
Figure 10. Van Gieson staining of leiomyomatous area showing black coloured nucleus, yellow cytoplasm and pink fibrocollagenous tissue (10x).
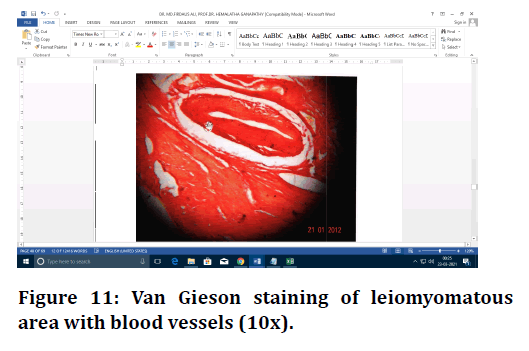
Figure 11. Van Gieson staining of leiomyomatous area with blood vessels (10x).
Discussion
The criteria for control group were hysterectomy specimens of hormonally not disturbed cases presented as prolapse uterus in the age group 35-65 years and specimens obtained from endometrial curettage performed along with cervical biopsy with no suppression of endometrial pathology with the age group 35-65 years. The study group included cases of DUB, Adenomyosis, Endometriosis and leiomyoma. The method used for the counting of blood vessels was that of Hourihan et al [4] and Sahasrabudhe et al [5]. The Endometrium in the control and study groups were dated as early proliferative, late proliferative, early secretory and late secretory. The blood vessels were concentrated more in basal layer in proliferative endometrium and were distributed more in the functional layer to late secretory phase which agreed with those of Fanger and Barker [6], Sahasrabudhe et al [5] and Nayha et al [7]. This is because the spiral arterioles are extremely sensitive to changes in levels of ovarian hormones and changes occurring in the vessel density as the cycle progresses. In fifteen cases of control, the average number of blood vessels were significantly higher in early proliferative phase in the basalis layer (4.0 ± 1.51).
The average number of blood vessels per 10 HPF in thirty-three cases of dysfunctional uterine bleeding were significantly lower as compared to control similar to that observed by Beazly et al [8] but in contrast to the findings of Abulafia et al [9], Makhija et al [2], and Nayha et al [7]. This is contradictory since there is a significant increase in endothelial cell proliferation in the endometria of women with dysfunctional uterine bleeding. The increased density of blood vessels combined with the fragile nature of these vessels maybe responsible for the bleeding in DUB cases.
Therefore, it is possible that bleeding in DUB maybe related to some qualitative changes in the blood vessels. It is also possible that the various morphological changes in the endometrial vasculature in DUB and rupture of dilated and congested vascular channels could be responsible for the abnormal uterine bleeding. In thirtysix cases of leiomyoma, the average number of blood vessels were significantly higher when compared with the control as seen in the study carried out by Farrer Brown et al [10]. This contrasts with the study by Sahasrabudhe et al [5] and Makhija et al [2] in which there was no significant difference observed in the mean of blood vessels in leiomyoma cases.
The ten cases of adenomyosis showed no significant difference in the average number of blood vessels as compared with control which contrasts with the finding of Beilby et al [11] and Makhija et al [2], in which adenomyosis cases showed reduced vascularity when compared to control. This is contradictory since adenomyosis is associated with abnormal uterine bleeding in a significant number of cases.
In the six cases of endometriosis, the average blood vessels per HPF were significantly higher (5.50 ± 0.70) as compared to the control. This agreed with that of Beilby et al [11] and Sahasrabudhe et al [5]. This is since in endometriosis essentially establishes a new network of blood vessels that supports the endometrial implant and allows the development of endometriosis. On comparison with four other studies mentioned, the present study is in agreement with those of Beilby et al [11] and Sahasrabudhe et al [5].
Conclusion
It is concluded from the present study that, the average number of blood vessels per 10 HPF were concentrated more in the basalis layer in the proliferative phase and were distributed more in the functionalis layer in the secretory phase. The average number of blood vessels per 10 HPF in endometriosis was significantly higher (5.50 ± 0.70) in leiomyoma and endometriosis when compared with the control. The present study shows a positive correlation between endometrial angiogenesis and uterine pathologies. In the present era of antiangiogenic therapies, endometrial angiogenesis and alteration in vascular morphology, this study has prognostic significance and may help to improve the treatment modalities and patient care.
Funding
No funding sources.
Ethical Approval
The study was approved by the Institutional Ethics Committee.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The encouragement and support from Bharath University, Chennai is gratefully acknowledged. For provided the laboratory facilities to carry out the research work.
References
- Hoozemans DA, Schats R, Lambalk CB, et al. Human embryo implantation: current knowledge and clinical implications in assisted reproductive technology. Reprod Biomed Online 2004; 9:692–715.
- Makhija D, Mathai AM, Naik R, et al. Morphometric evaluation of endometrial blood vessels. Indian J Pathol Microbiol 2008; 51:346-350.
- Sherman ME, Mazur MT, Kurman RJ. Benign diseases of the endometrium. In: Kurman RJ. Blaustein's pathology of the female genital tract, 5th ed. USA: Springer; 2002; 421-66.
- Hourihan HM, Sheppard BL, Bonnar J. A morphometric study of the effect of oral norethisterone or levonoregestrel on endometrial blood vessels. Contraception 1986; 34:603-12.
- Sahasrabudhe NS, Dalal SR, Jadhav MV. Endometrial blood vessels in health and disease. Med J West India 2000; 28:27-31.
- Fanger H, Barker BE. Capillaries and arterioles in normal endometrium. Obstet Gynaecol 1961; 17:543-50.
- Nayha V, Viitanen T, Stenback F. Altered extent, pattern and characteristics of microvascular density are indicators of neoplastic progression in the endometrium. Int J Cancer 2005; 115:975-80.
- Beazly JM. Dysfunctional uterine haemorrhage. Br J Hosp Med1972; 7:573-78.
- Abulafia 0, Sherer DM. Angiogenesis of the endometrium. Obstetr Gynecol 1999; 94:148-53.
- Farrer-Brown G, Beilby JOW, Tarbit MH. The vascular patterns m myomatous uteri. J Obstet Gynaecol Br Commonw 1970; 77:967-75.
- Beilby JO, Farrer-Brown G, Tarbit MH. The microvasculature of common uterine abnormalities, other than fibroids. J Obstet Gynaecol Br Commonw 1971; 78:361-8.
Author Info
Firdaus Ali and Hemalatha Ganapathy*
Department of Pathology, Sree Balaji Medical College & Hospital Affiliated to Bharath Institute of Higher Education and Research, Chennai, Tamil Nadu, IndiaCitation: Firdaus Ali, Hemalatha Ganapathy, Morphological Study of Endometrial Blood Vessels , J Res Med Dent Sci, 2021, 9(6): 150-158
Received: 08-May-2021 Accepted: 16-Jun-2021
