Review Article - (2022) Volume 10, Issue 11
Mucormycosis and Its Fate in COVID Infected Patients
Purvi Tiwari* and Swarupa Chakole
*Correspondence: Purvi Tiwari, Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical, India, Email:
Abstract
Background: The world has seen an endemic like never before, the disease which is devastating and destructing to human mankind. COVID-19 infection (SARS-Cov-2) spreads like a web all over the world and when the whole world was busy in fighting with COVID, another deadly infection i.e. Mucormycosis was making home. Mucormycosis a rare but life threatening fungal infection produced havoc in our country. Its actual cause is unknown but many theories and evidences have been given which states that its probable cause might be the overuse of steroids in moderately to severe COVID infected patient. Steroids lower down the immune response of the body thus, giving shelter to such opportunistic infection. Along with steroids, diabetes being a high risk factor for its development. In COVID infection there is high blood sugar level due to broad antibiotic coverage, steroids use and long term stress. The surge in the COVID cases along with mucormycosis cases increases the mortality and morbidity in COVID-19 patients. As it was a rare disease, its pathological course and its sequels is still unknown, as large studies and trials were impossible to perform.
Summary: The overuse of steroids and high blood sugar levels remains the big risk factor for the development of COVID-19 infection as the immunity is already weakened. And it’s associated morbidity and mortality remains constantly high.
Conclusion: There’s not much studies has been conducted but patients progresses after giving Amphotericin B. Antifungal agents and surgical interventions remains the main stay of treatment and insulin to keep blood sugar level under check.
Keywords
COVID-19, Mucormycosis, Steroids, Diabetes, Immunity
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS COV2), a transmissible respiratory infection which emerged in year 2019 in Wuhan city, China. After which it continues to spread till date and becomes a global pandemic. The COVID cases surges day by day due to its airborne route of transmission and this rise in cases can only be inhibited by preventing the virus entry inside the respiratory tract.
Symptoms and signs are also sometimes mild which makes it more difficult to diagnose this includes mild fever, cough, cold and body ache which can turn into life threatening severe pneumonia to acute severe respiratory failure. In humans, the pathogenesis of SARS-CoV-2 begins from multiplying in the upper respiratory tract to travelling through the airways after attaching to epithelial cells in the respiratory system. It then reaches alveolar epithelial cells in the lungs. SARS CoV-2's proliferation in the lungs may elicit a significant immune response which results in massive cytokine storm. Acute respiratory distress syndrome and respiratory failure are the most common causes of death in COVID-19 patients, due to cytokine storm syndrome. Patients over 60 years old and those with major pre-existing conditions are more likely to suffer acute respiratory distress syndrome and ultimately leading to death [1].
Literature Review
Mucormycosis
Due to the virus inside the body immunity becomes weaken and antibiotics and steroids being the main stay for the treatment further leads to a body susceptible for fungal infection. Capability of pulmonary macrophages due to high viral load is also lost and fungal species which enters the lung can’t be eliminated. Mucormycosis refers to a group of illnesses caused by fungi known as Zygomycetes, which produce branching fungi hyphae that look like ribbons and reproduce sexually by forming zygospores. Pathogens can be discovered in a wide variety of fruits, it can also be cultured from the oral cavity, dirt, and faeces. Zygomycetes has a subtype called Mucorales which provides a distinct clinical infection pattern.
Generally Mucorales attack deep tissues most effectively through eating or inhalation of spores, as well as percutaneous injection of spores. Even if it gets into the lungs or skin, the first line of defence in a healthy organism is to use oxidative stress to kill the spores as it does not have strong virulence property so, it can’t produce disease in a healthy individual. Thus mucormycosis needs comorbid conditions to develop a disease inside the body. And in the presence of cationic peptides and metabolites this becomes a source of worry (Figure 1).
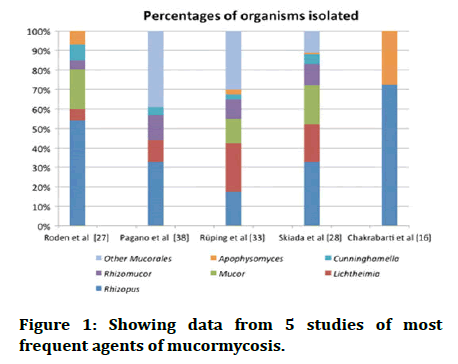
Figure 1: Showing data from 5 studies of most frequent agents of mucormycosis.
Predisposing conditions and risk factor
Diabetes, especially ketoacidosis, when the body is on steroids, old age, and other chronic conditions like AIDS, neutropenia or other haematological malignancies, renal failure, etc. if you've had an organ or stem cell transplant, if you've had an iron overload, long term broad spectrum antibiotic given, intravenous drug abuser are some comorbid conditions in which disease can progress [2]. Sometimes it can also occur in patients with normal immune system which can be related to burns, trauma and iatrogenic factors, contaminated bandages and dressings, central venous catheters.
Diabetes being the most common risk factor for the development of the disease, associated with around 36%-88% cases, it modulates the overall immune response of the body, high blood sugar level favours the growth of fungus, ketone bodies are effectively used by the fungi, it also decreases the chemotactic and phagocytic efficiency of the body. Ketone reductase, which is generated by Rhizopus species, also helps in rising the glucose and acidic environment in patients with DM. There is a risk of developing any sort of Mucormycosis, especially if they have ketoacidosis. Neutrophil plays a vital part in the host defence system against Mucorales, thus when a host is infected with DM, its function is hindered at each level of DM. Furthermore, ketoacidosis in diabetes has been shown to hasten fungal invasion. Diabetics with a low amount of dialyzable inhibitory factor and an acidic back ground produces more free iron by lowering its binding to transferrin and making free iron more available to the fungus which helps in its proliferation and maturation (Figure 2).
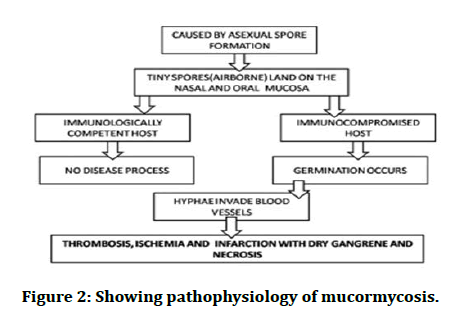
Figure 2: Showing pathophysiology of mucormycosis.
Chronic corticosteroid based therapy increases a patient's susceptibility to mucormycosis through producing abnormalities in macrophages and neutrophils, and it can also produce hyperglycaemia from over usage of steroids. As steroids are the mainstay for the treatment of COVID-19 infection which makes it a primary risk factor for the development of mucor. The infection appeared to show in any clinical form in the few cases of mucormycosis with systemic lupus erythematosus, but disseminated mucormycosis was widespread and the mortality rate was very high 88%. Patients with various autoimmune disorders may develop opportunistic mucormycosis. Mucormycosis can be misdiagnosed in people with Wegener Granulomatosis because it mimics a return of the underlying disease [3].
Angioinvasive mucormycosis has been linked to the use of DFO, an iron chelator used to treat iron and/or aluminium excess in dialysis patients. According to a data from an international registry of mycoses, DFO was given to 78% of dialysis patients with mucormycosis. Iron overload is also an important risk factor, whether from transfusions or dyserythropoiesis, high levels of available iron helps in the maturation of the fungus. The most prevalent form of mucormycosis in patients receiving DFO appears to be disseminated mucormycosis 44%, which is associated with significant fatality rates of up to 80%.
Mucormycosis is very uncommon in people who have the Human Immunodeficiency Virus (HIV) or AIDS. HIV being the immune compromised state has lower frequency of mucormycosis cases which may be explained by the disease having relation with neutrophil deficiency and has nothing to do with the T lymphocytes.
Mucormycosis accounts for a few percentage of invasive fungal infections in Solid Organ Transplant (SOT) patients, but it is linked to a high death rate. Depending on the type of organ transplant, the predicted incidence varies from 0.2% to 1.2% in renal transplant patients, 0% to 1.6% in liver transplant recipients, 0% to 0.6% in heart transplant recipients, and 0% to 1.5% in lung transplant recipients. All of the patients were on a long term immunosuppressive regimen, which included large doses of systemic corticosteroids. Furthermore, mucormycosis spread to distant organs often following rejection and therapy. Dissemination took place with a preference towards skin and soft tissue, sparing brain. Compared to other SOT patients, liver transplant recipients were more likely to develop disseminated illness and mucormycosis sooner after transplantation.
Clinical manifestations
According to anatomical localisation, manifestations of mucormycosis can be classified as
• Rhino cerebral.
• Cutaneous.
• Pulmonary.
• Gastrointestinal.
• Disseminated.
• Uncommon presentations.
Rhino cerebral mucormycosis
It has three subtypes depending on the structures involved
• Rhino cerebral
• Rhino orbital
• Rhino maxillary
Among all the types it is the most lethal form and most common in diabetics, especially in individuals with diabetic ketoacidosis. Infection occurs after the inhalation of spores with some comorbid conditions. Spores gain access to nasal and oral mucosa then germinate into hyphae and then can invade the blood vessels which can lead to edema ischaemia, infarction, necrosis and dry gangrene of soft tissues, because of rapid invasion it can produce distant infections of brain and other organs. Symptoms initially are non-specific and include fever, headache, nasal obstruction and discharge, crusting and facial pain which gets worsens within few hours or days to specific symptoms like unilateral headache, facial edema, back discharge, blurring of vision to complete vision loss. Due to involvement of other structures it can lead to further complications like: Sinusitis, Cellulitis of the orbit, orbital apex syndrome, Cavernous sinus syndrome, Hemiparesis due to thrombosis of the internal carotid artery, Cranial nerve involvement, Features of central nervous system involvement.
Stages of rhino cerebral mucormycosis
Stage 1: Fungal invasion into sinonasal mucosa and infiltration into surrounding blood vessels accompanied by thrombosis of the affected blood vessels, as well as necrosis that is rapidly spreading to the surrounding soft tissues, such as the skin of the face and the mucosa of the mouth.
Stage 2: Infection moves to the orbit via direct and/or vascular invasion. As a result, at this stage of the infection, eye discomfort, chemosis, proptosis, ophthalmoplegia, and even blindness might develop.
Stage 3: Invasion into the orbital apex, cribriform plate, and fovea ethmoidalis. By this time, the infection has manifested itself in the form of cognitive symptoms such as confusion, obtundation, and can also lead to death (Figures 3 and 4) [4].
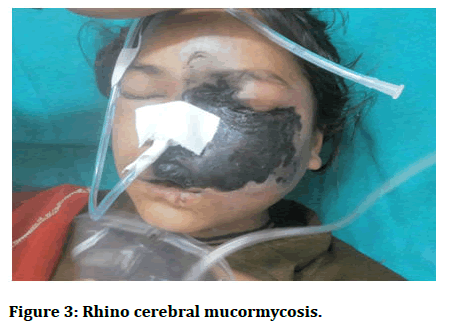
Figure 3: Rhino cerebral mucormycosis.
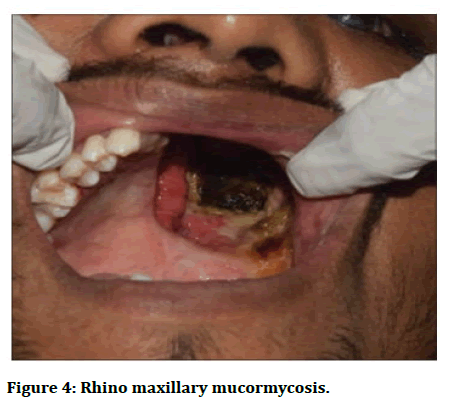
Figure 4: Rhino maxillary mucormycosis.
Cutaneous mucormycosis
It has been divided into primary which occurs due to the direct inoculation of fungus into the skin and secondary mucormycosis which occurs due to the dissemination of the disease. It is further subdivided into localised, deep, disseminated according to the extent of disease. The onset of primary cutaneous mucormycosis might be gradual or fulminant. The clinical picture isn't always the same. Lesions begin as indurated plaques that range in colour from erythematous to purple. These turn necrotic and create an erythematous halo, which can turn into an eschar. Targetoid lesions, sensitive nodules, ulcers, purpuric lesions, and swollen and scaly plaques are some of the other symptoms. Cellulitis and necrosis are common in patients with surgical wound infections and burns [5]. Secondary cutaneous mucormycosis is more common than primary illness. It has a rapid onset and a high fatality rate. Necrotic eschar is the most common cutaneous finding (Figures 5 and 6) [6].
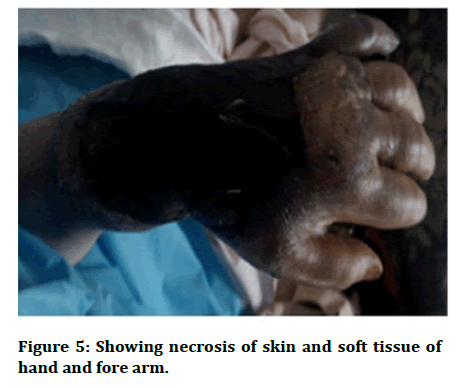
Figure 5: Showing necrosis of skin and soft tissue of hand and fore arm.
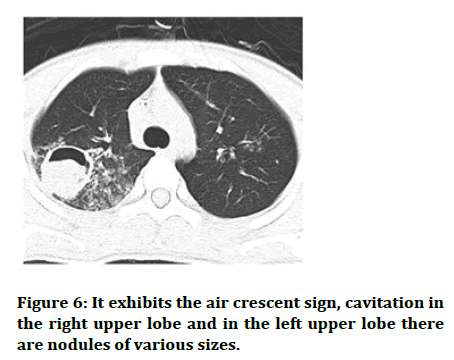
Figure 6: It exhibits the air crescent sign, cavitation in the right upper lobe and in the left upper lobe there are nodules of various sizes.
Gastrointestinal mucormycosis: It is generally considered to be rare and is secondary to the ingestion of fungi. The stomach, colon, and ileum are the most prevalent sites of gastrointestinal mucormycosis and stomach being the most common. GI mucormycosis has also been reported in conjunction with other immune compromising illnesses such as AIDS, systemic lupus erythematosus, and organ transplantation in extremely rare occurrences. The features of gastrointestinal mucor cases vary depending on the site of infection. The most common symptoms are nonspecific pain in the abdominal and abdominal distention, as well as nausea and vomiting. Fever and hematochezia are also possible side effects. There has also been an intra-abdominal abscess discovered [7-10].
Disseminated mucormycosis: Disseminated variety of mucormycosis is not very common to see. Infection from the primary focus goes into the blood stream and spreads to other parts and organs of body, among disseminated variety brain is the most common type, others being heart, spleen, skin [11].
Miscellaneous manifestations: Mucorales agents have the ability to infect almost any part of the body. Among the absence of sinus infection, endocarditis, and pyelonephritis, brain involvement occurs on rare occasions, primarily in intravenous drug addicts. Some cases of mucormycosis of bones, mediastinum, trachea, kidneys, and peritoneum have also been reported [8].
Prevention of mucor in COVID patient: The underlying risk factors for COVID associated mucormycosis must be addressed in order to prevent the disease.
• Aiming for the better control of blood glucose level in diabetics,
• Proper use of systemic corticosteroids,
• Systemic corticosteroids should be used correctly.
Avoiding the use of antibiotics, antifungals, and immune modulators that aren't needed. Proper care of equipment’s used by numerous patients and hygiene should be maintained, Wound care that works (bandages, tapes used for endotracheal tubes, ostomy devices should be sterilised properly and changed on a regular basis), in health care institutions, proper line management is essential.
Discussion
The burden of this fungal illness can be decreased by good care along with early diagnosis and the use of antifungals drugs, followed by the use of superior therapeutics in the initial stages of the disease to prevent more disastrous consequences [10]. Prevention in immune compromised and proper care of secondary infection with early diagnosis is the key for the successful treatment outcome. Antifungal treatment and surgical debridement are the only two treatment modalities we know till date. Antifungal therapy includes drugs like Amphotericin B, Posaconazole, or Isavuconazole. Amphotericin B, Posaconazole, and Isavuconazole are administered intravenously and antifungals which can be given orally in mucor cases are Posaconazole, Isavuconazole. Fluconazole, Voriconazole, and Echinocandins are among the medications that do not function against the fungus that cause Mucormycosis [12,13]. The only antifungal medication approved for the treatment of Mucormycosis is Amphotericin B Deoxycholate (AmB). The Lipid Formulations of Amphotericin (LFABs), on the other hand, are much less nephrotoxic than AMB and can be safely supplied at higher doses for longer periods of time. For the treatment of Mucormycosis, LFABs appear to be safer and more effective than AmB. The therapy of choice is liposomal Amphotericin B at a starting dose of 5 mg/kg body weight (10 mg/kg body weight if CNS involvement is present). There are 50 milligrams in each vial. Normal saline incompatible with it, hence it should be diluted in 5% or 10% dextrose. It must be maintained for many weeks until a strong response and disease stabilisation are reached, after which oral Posaconazole (300 mg delayed release tablets twice a day for 1 day, then 300 mg daily) or Isavuconazole (200 mg 1 tablet 3 times day for 2 days, then 200 mg daily) can be used [14]. Antifungal treatment is continued till the resolutions of symptoms and till the active disease becomes radiological inactive. Mucormycosis has a host and site dependent response to antifungal medications, which is particularly problematic in individuals with haematological abnormalities and HSCT recipients [8]. Surgical intervention is needed when the infection becomes aggressive or it was diagnosed in later stages of the disease, surgical debridement of all dead and contaminated tissue, it should be removed as soon as possible. Because the palate, parts of the nose, and parts of the eye may be removed during surgery, it can result in disfigurement. However, without such severe surgery, the prospects of survival are low [13]. Mucormycosis consequences are usually connected to the area of the body that was infected, but they can also occur in other parts of the body since the fungus typically spread to organs or tissues that come into direct touch with or are near the affected area. This includes blindness, pulmonary and gastrointestinal haemorrhages, meningitis, brain abscess, sepsis. To prevent these complications early initiation of the treatment is needed [15].
Conclusion
Mucormycosis is a fungal infection which occurs as a secondary infection or in immune compromised state and is most commonly associated with corona virus infection. COVID-19 infection itself leads to immune compromised state but its mainstay treatment that is steroids further leads to more susceptibility to mucor fungus. Thus, mucormycosis is the most common secondary infection in COVID-19 recovering patients. Host susceptibility increases more when there is preexisting debilitating conditions like hypertension, diabetes, cardiovascular diseases, haematological abnormalities, organ transplanted patients, out of which diabetes being the most important and the most prevalent risk factor for the occurrence of the disease. And by strict glycaemic control, disease occurrence can be prevented. It has different clinical manifestations and the disease can spread to any site of the body. Rhino cerebral mucormycosis is the most common presentation seen. As the disease has sudden onset and rapid progression, early detection of the disease is important for which high resolution CT scan plays a vital role, culture biopsy can also be perform for the confirmation of the diagnosis. Because of its rapid progression and variable manifestations and different host response to anti-fungal, the outcome becomes highly unpredictable with increased rates of mortality. Surgical debridement of the diseased tissue or part or organ becomes necessary for the survival of the patient along with the use of antifungal therapy. IV Liposomal Amphotericin is the antifungal of choice as it is least nephrotoxic and more effective, after symptoms subside oral Posaconazole can be started. The prognosis of the disease is poor thus prevention from the disease can only save the patient from complications.
References
- Hu B, Guo H, Zhou P, et al. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021; 19:141–54.
- Nishanth G, Anitha N, Aravindha Babu N, et al. Mucormycosis a review. Eur J Mol Clini Med 2020; 7.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis 2012; 54:S23–34.
- NEWS Medical Science Life. What is Rhinocerebral Mucormycosis? 2021.
- Castrejon Perez AD, Welsh EC, Miranda I, et al. Cutaneous mucormycosis. An Bras Dermatol 2017; 92:304–311.
- Wang X, Guo L, Xue S, et al. Pulmonary mucormycosis: A case report and review of the literature. Oncol Lett 2016; 11:3049-3053.
- Spellberg B. Gastrointestinal mucormycosis: An evolving disease. Gastroenterol Hepatol 2012; 8:140-142.
- Spellberg B, Edwards J, Ibrahim A. Novel perspectives on Mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev 2005; 18:556-569.
- Centers for Disease Control and Prevention. Mucormycosis. Department of Health and Human Services. U.S.
- Boretti A. Steroids induced black Fungus infection in India during the May 2021 COVID-19 Outbreak. Indian J Otolaryngol Head Neck Surg 2021; 1-4.
- Centers for Disease Control and Prevention. About Mucormycosis. Department of Health and Human Services. U.S.
- Centers for Disease Control and Prevention. Treatment for Mucormycosis. Department of Health and Human Services. U.S. 2021.
- Mediline plus Trusted Health Information for you. Medical Tests. Department of Health and Human Services National Institutes of Health. U.S.
- Davis CP, D Nettleman. Guideline for management of Mucormycosis in COVID-19 patients. 2021.
- Chandrasekar PH, Sandhu A. Medscape. Mucormycosis (Zygomycosis). 2021.
Author Info
Purvi Tiwari* and Swarupa Chakole
Department of Community Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical, IndiaCitation: Purvi Tiwari, Swarupa Chakole, Mucormycosis and Its Fate in COVID Infected Patients, J Res Med Dent Sci, 2022, 10 (11): 069-073.
Received: 02-Sep-2022, Manuscript No. JRMDS-22-64692; , Pre QC No. JRMDS-22-64692(PQ); Editor assigned: 06-Sep-2022, Pre QC No. JRMDS-22-64692(PQ); Reviewed: 21-Sep-2022, QC No. JRMDS-22-64692; Revised: 03-Nov-2022, Manuscript No. JRMDS-22-64692(R); Published: 10-Nov-2022
