Review Article - (2022) Volume 10, Issue 9
Peri-Implantitis-An Overview
Tanishka Taori*, Bhairavi Kale and Khushboo Durge
*Correspondence: Tanishka Taori, Department of Periodontics, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences DMIMS (Deemed to be University) Sawangi (Meghe): Wardha 442001, Maharashtra, India, Email:
Abstract
Peri-implant inflammations are severe disorders that affect both the external and internal tissue after dental implant therapy. Dental implants have emerged into a reliable method for restoring single or multiple lost teeth over the last 25 years. Despite the expected treatment outcomes, with the majority of implant failures happening during early healing and the first year of loading, complications do arise during implant care and retention. The cause of mucositis is due to the plaque which is between the implant and soft tissue interface. The peri-implant soft tissue contact is less effective at resisting bacterial invasion than natural teeth, increasing the risk of peri-implant illness Furthermore; it can proceed to inflammation of an implant in certain people, resulting in demineralization and fixture failure. These treatments include various manual ablations, laser assisted devices and therapies which use photoactive dyes, which can supplement with medications, either local or systemic. Osseo integration can be re-established. Therapeutic approaches seem to be more likely than standard treatments. In cases of advanced peri-implantitis, depending on the defect's on configuration resective surgery can be used to eliminate peri-implant lesions, while regenerative therapies can be used to fill the defect. The Cumulative Interceptive Supportive Therapy (CIST) regimen is used to guide peri-implantitis treatment. The primary goal is to provide an overview about the risk factors, ethology, and pathogenesis, clinical and radiological features of periimplantitis and is to present an up to date, current and brief evidence based assessment of peri-implant illnesses and disorders, as well as to address current treatment approaches to primary care and recent improvements in disease prevention. Implants have evolved into a very dependable perioperative period for restoring single or many lost teeth over the span of decades.
sushihouse kolstads americanpridefasteners trueforge hotelposeidon youngswoodfinishing arditomason thebestofreno eeclongisland doktervanhecke famsales adla bogatylaw prefplastics kirbycontractinginc unlsp boban dottoressaromolimonica thebestofsanfrancisco vernix digitaldocuments tristatepropertybrokers sotrafib cemcorpny agenziaimmobiliarebuti joesitalianfoodmarket corpoguardiedicitta icsatc antoniniassicurazioni besel strappedincarseatsafety drevobeton blessingconstructionny centre-endorphine qmbtunisia elearning 357 mpress rrappliance ciaautomazioni antikva stubbanvel tacticalpublicrelations mpulshnk paulyboybrand lordshoes ijd-procom thebestrestaurants atkpalvelut ceat leonfukspc pilotexamssa ashgrovecabins universalshielding thebestofcharlotte johnjmazurinc rollnroaster thecabinetwarehouse pocketchangeduo thebestofmilwaukee resan sooli vwe centralwindowcleaning reinforcedplasticslab bugbustersofli ciandrigiardini arfada centrumeigenwijs thebestofmemphis maisonpearly islipll dcgraphicsinc agetranquille relisandroth thebestofcharleston traiteur-wn safi-ingenierie justmyvoice harrishardware louisbarbatolandscaping brightstartoursworld rga-insurance calacatajumper ezantia mrcheapocds van4holiday cigap richtour fagyhatar sicc weshopmall ventovuori sobatrapcapbon unityrubberllc thebestofsaltlakecity ircinc sescoindustries connply storen-servicesenter psnry greatneckcollision ironfitendurance tuscanycountryhouse sansovinocalcio bbradydesign samsam vdtarification springersoil thebestofdayton milantechnology labradoodlesoflongisland daliamohamed theconsultantpowerhouse gunnbrush kruunuosk shulmanproduce balcosupply conso-med federalnetworks bellmoreglass alwaysaffordableconcrete jayteeinsurance carolina-cabins cmorfinance stretchritepackaging dishaairwaysenterprise biocontrol distribio peterivill lcc inspekta jvidesigns royalroseinc calabriapizza fadhila prato-pronto-effetto arbemachine olsonelectricnj tkbl tuttifotokft alpernmd ggsupplywholesale almaxcorporation sotuflex ecustomgutter studenipotok metal-lineconcept rettsodontologi nutecsystems aclotbeach gjonnes-bygg cavalierinternational mnemos southfloridashuttles ajchemicalsupply rachelsfireisland concepto nesponge ultimatestylesofamerica techniquesmadeeasydrivingschool stevesmeatsfreeport eastwest gritbrush plussplan biosens viltkam wikaya paintballconcept justmyvoice securecarkeysupply justmyvoice allcountylegal catt plugandcharge ttandlcontracting justmyvoice islandboatlettering gms-tunisie sportsiena bloeiop touchofclasscollision kotekservice ittoscana pallongislandlacrosse industrialfinishings painoutband rakvag-batforening konemies rrfamilychiropractic infienile shbcgroup footpharmacydirect scuolaguidaprato ans-nettoyage autoskola saafa autolaky1 fixcars longislandelitelandscaping rayscan palaconstruction studio44 crealhome viniferi gavinburke ciprianigiardini davidpokorny osteriailcapodaglio thebestoffairfax centroorafofaccioli ateliervb bbdps thebestoffresno prodigus stratpak umisushirestaurant sotim suldalrenovasjon amer-equip k-kleven jerryspridepotatoes neuroky psicologozampoli jjslandscaping thebestofoklahomacity responsivesales paratie-antiallagamento-shop hearproof cfat ruspinameubles planetbioplastics lynbrook-plumber fcdf-ye brechanparkett valley-stream-plumber diagnosismaker aquavaria jedit garageennour heimdalbygg thebestoflittlerock raybomarine bayshorepaper gmstowing potatura-abbattimento-piante thebestoflouisville michaelalbert thebestofannarbor unitypavers mtnfueloil durub-mudiya lesgensdere dormerking thusney italianvistatravel maiemad gtiuniformcleaning suddenimpactli levituuli sourisalavie mayoiltank mschwartzfeather rands arieslimousines orthoticworld longislandcocktailhours waterjet allislandpaving dukediagnostic klfgoteborg yanezviaggi apruk decogato springeroilltd semapsolar irisgioiellicomprooro msedpsoftware fourcmanagement holemans 7consulting medinet evertile robertwitcomblandscape ceramics adrobotengineering volt-energy huisjacobs chimneyserviceboston eastmainstdental gcbt rememberingbriank elligiardiniespurghi paulslandscaping luisrestorations mgstunisie fixcarsny crcdd hotelprincipessalucca turvahallinta ralphjr thales irrigazione-giardini spantecsystems biomedic fgt-trading sols-egypt spectrumlaboratoriesinc prosecurite spongewarehouse afsainc dellafrancadevelopmentgroup leragazzedifirenze ferrettiwatches dovreentreprenor digitalhvac mostlymica fleurs-velghe autoscuolalebadie interlockingrubbertiles meadowcreekhoa bcn corpjetsupport jrkitchensflooring arteletti
Keywords
Peri-implantitis, Peri-implant mucositis, Cist, Risk factors, Etiology, Pathogenesis, Clinical and radiological features, ManagementIntroduction
A dental implant or a fixture is a surgical component that connects with the jaw or skull bone to anchor a dental prosthesis such as a crown, bridge, denture, or facial prosthesis, or to act as an orthodontic anchor.Implants have evolved into a highly dependable surgical technique for resolving single or multiple tooth loss [1]. Osseointegration is a continuous process that links the functioning of osteoclasts and osteoblasts in the processes of bone healing, bone production and bone adaptability for improved function [2]. The efficacy of an implant is determined mostly by the degree of Osseo integration accomplished, but also by its optimal allocation and natural-looking replacement [3].The implant's growth is based ascertained by its continuity, which is established by the following factors: surgical technique, bone quality, quantity, implant shape and surface topography qualities, all of which can be modified as needed to achieve strong primary reliability and protracted implant therapies accomplishments [4].
Dental implants also transformed dental loss rehabilitation towards the point where they are now the norm of practice in a variety of scenarios [5]. Dental implants are used in dentistry for the replacement of lost teeth in a variety of clinical scenarios. A rate of success for implant after 16 years of follow-up is 82.9% which have been reported [6].Despite the increased treatment efficacy of dental implants, they can fail, experiencing discomfort and broad oral loss. Surgical rehabilitation of a failed implant, which is painful and costly. Inflammation of the soft and hard tissues in the site of a working implant surface [7]. Peri-implantitis is characterised by inflammation in the preimplant connective tissue and progressive bone loss [8]. Dental implant related condition that is becoming more common in clinical settings, contributing to a sizable proportion of implant failure which contains posterior maxilla, has the highest performance degradation due to poor bone quality and insufficient bone volume, which is connected to both the size of the maxillary sinus and alveolar ridge degradation [9]. As contemporary dentistry progresses in advancements, the focus was on implant design on both a macroscopic and nano scale to optimize and make it successful [10]. Preimplant diseases are mainly categorized into two types i.e. peri-implantitis and peri-implant mucositis depending upon the severity. The absence of signs of inflammation and leaking on Probing (BOP), increased penetrating level (PD) and diagnostic bone resorption are now all hallmarks of bone turnover is indicative of peri-implant health (RBL). Peri-implant mucositis is distinguished not only by the presence of visible signs of inflammation/BOP, but also by the absence of increased PD and RBL. Peri-implantitis is diagnosed by the presence of signs of inflammation/BOP with increased PD and RBL beyond anastomotic threshold; and RBL greater than anastomotic threshold. The factors which can aggravate the peri implant diseases are smoking, systemic disease (like cardiovascular disease, Diabetes mellitus and immunosuppression), iatrogenic causes like cementitis, etc. As a result, strategies for peri-implant disease prevention and treatment should be incorporated into modern rehabilitation concepts. The current review provides an up-to-date overview on the treatment and therapy option [11].
Literature Review
Risk factors and etiology
Implant loss within one year is “EARILER IMPLANT LOSS” and if it takes more than one year it is “DELAYED IMPLANT LOSS". Risk factors include:
Systemic diseases: Anaemia of Chronic illness (ACD) is a decrease in Hb, and there is a link between peri-implant disease and ACD. As a result of the local inflammation, there was a considerable increase in WBCs, platelets, RBCs and mean corpuscular haemoglobin concentration. Serum biochemical parameter that is also known as CVD Markers results in higher level of triglycerides, uric acid and WBCs in patients with peri-implantitis. There seems to be an elevation in proinflammatory mediators (e.g. IL-6 and IL-10) in the GCF under this scenario [12]. Diabetes mellitus is a set of metabolic disorders 2 leads to the deleterious changes in periodontium and failure of host defence mechanism impaired wound healing, a protracted inflammatory response, microvascular changes and a reduction in new bone formation and repair. Hyperglycemia has also been linked to a delay in wound healing, which supports the idea of enabling diabetic patients to have longer osseointegration times [5].
Periodontal diseases: Low total bacterial loads, particularly gram-positive cocci, characterise healthy peri-implant biofilms (resembling actual teeth), resembling actual teeth, with increasing total bacterial load and in particular, higher proportions of Gram positive and gram-negative bacteria and also with red complex species. An actinomycetemcomitans and P gingivalis are the main microorganisms in this disease [5].
Smoking:Cigarettes was shown to impede the endogenous aggressive regeneration by diminishing proinflammatory chemoattractant sensitivity, locomotor action and oxidative antibacterial pathways and that hyper inflammation is present in both diabetic and established periodontal disease. The immunomodulatory effects of smoking, whether pro-inflammatory or anti-inflammatory, may cause mucosal inflammation to persist [5].
Titanium corrosion: when the salivary pellicle layer is formed on the implant surface HMW mucins, salivary enzymes were found in salivary pellicles on titanium. Studies using Polymerase Chain Reaction (PCR) and Deoxyribonucleic (DNA) hybridisation revealed that molecules like LMW mucins were not detected [12]. Bacteria create acidic toxins, which cause the environment to become acidic. The titanium oxide layer may be disrupted or dissolve as a result of this. Furthermore, fluoride is added to commercial toothpastes and gels to prevent dental cavities and so the fluorides dissolve titanium oxide and it corrodes in presence of acid. Implant corrosion can be the result of mechanical wear is also exacerbated by chemical and biological wear [13].
Genetic factors: Crevicular fluid peri-implant TNF-alpha could be a sign of peri-implant inflammation. IL-1 is a family of 11 cytokines that have a role in immune system regulation it’s linked to peri-implantitis and inflammatory reactions [5].
Residual cement:Stock supported implants that do not meet the gum contours make adjacent teeth cement removal difficult or impossible and accessible margins might create aesthetic concerns [5].
Occlusal overloads: Occlusal overloading leads to bone loss induced directly by micro injury and it was linked to peri-implant marginal bone loss [5].
Poor plague control: The patient's competence to systematically maintain the fixture site with brushes, interdental brushes, and floss may be obviated by implant prosthesis design. As a result, it is the responsibility of dental providers to educate patients about correct plaque control and to guarantee that periodontal maintenance is established on a regular basis. This will aid in determining whether plaque removal was effective [5].
Keratinized mucosa: In comparison to implants encircled by a KM of 2 mm, preimplant hard tissue injury was seen [8].
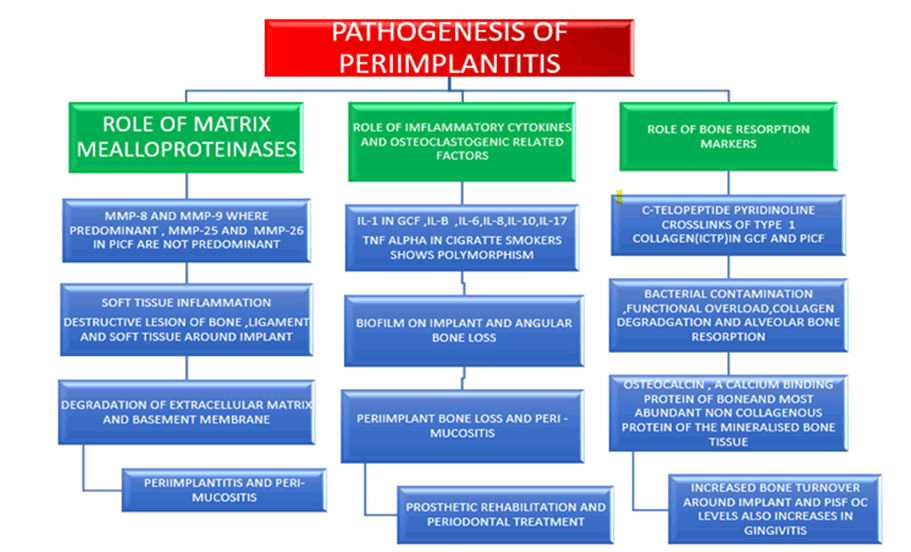
Previous history of implant failure: Microbial colonisation is the key predisposing factor in the aetiology of peri-implant disorders. Glycoproteins from saliva bind to exposed titanium surfaces as soon as they are exposed to the mouth cavity, resulting in microbial colonisation. Biofilm surrounding implant plays an important role in the initiation and progression of peri-implant disease (Figure 1). Furthermore, in patients with severe chronic periodontitis (Figure 2), peri-implant infections have been linked to gram negative anaerobic bacteria identical to those found around natural teeth. When peri-implantitis is detected late, there is a complete loss of “osseointegration”, which necessitates removal of implant. Unlike" initial failure" contaminants during installation or reduced bone restructuring even during medical reconstruction phase of treatment, peri-implantitis is a slime-induced condition of already Osseointegrated implants "delayed failure" [10].
The etiological factors which cause peri-implantitis are:
Local factors
- Peri-implantitis
- Position of the implant site
- Bone quality and quantity
- Irradiated bone
Technical factors
- Different implant surgeries
- Length of implants
- Mechanical failure
Pathogenesis
Figure 1: Pathogenesis of peri-implantitis.
GCF- Gingival Crevicular Fluid
PCIF- Peri Implant Sulcular Fluid
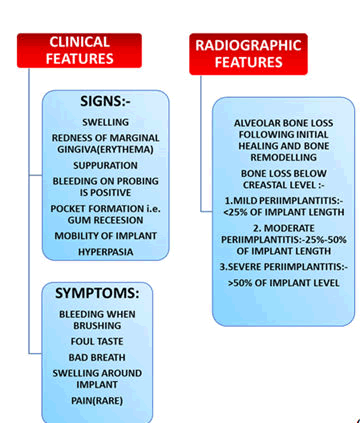
Figure 2: Signs and symptoms of clinical and radiographic features.
Peri-implantitis: Treatment options
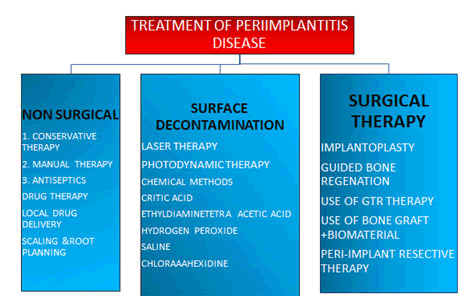
Implants that ailing or failing have a variety of treatment options. The overarching goal of therapy is to achieve a functional and aesthetically pleasing restoration. As a result, all treatment should be aimed at stopping the disease and restoring a healthy peri-implant mucosal barrier [14,15].If identified early enough, peri-implant mucositis can be successfully treated with nonsurgical therapy (Figure 3), whereas peri-implantitis usually necessitates surgery [16].
Figure 3: Treatment of peri-implantitis disease.
Non-surgical interventions
Conservative therapy: Mechanical debridement, occlusal therapy and pharmacological therapy are all used in this form of treatment. The first line of treatment for an ill or failing implant is mechanical debridement. To avoid damaging the titanium surfaces, plastic, graphite or nylon instruments, as well as those with a Teflon coating, are advised. To remove endotoxin citric acid is used and chlorhexidine is used for the antimicrobial effect. Mechanical cleaning is intended for implant restorations with PD<3 mm and plaque and/or calculus, whether or not BOP is present and also focused on plaque removal with a Prophy cup and scaling calculus. The roughened surfaces of most implants, which encourage colonisation and bacterial adherence, make mechanical debridement difficult. Steel curettes or ultrasonic equipment should not be used on the implant surface because they can cause the surface to change, allowing germs to colonise. Peri-Implantitis can be treated with a sonic or ultrasonic-driven Poly Ether Ketone plastic tip (PEEK) [16]. The surface of implants can be modified using an abrasive air polishing medium. Cell attachment and survival remained adequate after air powder treatment; however, cell responsiveness was reduced.
Drug therapy
- Antiseptic rinses
- Systemically and locally delivered antibiotics in relation to pocket depth or different parameters.
Antiseptic therapy: is indicated for lesions with PD of 3 to 5 mm, EDTA, citric acid, H2O2, LA are used to eradicate the biofilm.Through use of mouthwash resulted in a decline in crevice levels and an antiplaque impact. It was a toxic reagent that inhibits gram-positive and Gram-negative germs [17].
Antibiotics: Such as tetracycline, amoxicillin, sulfonamides + trimethoprim etc. were used locally and resulted in considerable reductions in pocket depth [6]. If local antibiotic drug carriers remain just at targeted site for a minimum 7 to 10 days, these appear to become as efficacious as centrally delivered drugs, with the additional benefit of eliminating the negative events associated to antimicrobial drugs [5]. Amoxicillin had different growth-inhibiting effects on S. sanguinis, P. gingivalis. The antioxidant grapefruit juice showed just a bacteriostatic impact on Streptococcus aureus [6].
Anti-calculus agents: Dentifrices containing calcium phosphate are shown to be highly effective in preventing the build-up of slime. Calcification of the teeth enzymatic dissolution is caused by mucinase. In calculus, organic matter is represented by pyrophosphate, whereas pyrophosphate is represented by pyrophosphate and prevents the formation calculus. The amino acid buffered hypochlorite solution comprises 0.95 percent sodium hypochlorite, as well as amino acids including glutamic acid, leucine and lysine, which are used to treat damaged implants and Poly pyrophosphate anion in dentifrice also acts as anti-calculus agents [6].
Manual treatment: Teflon, carbon, plastic and titanium curettes can provide basicmanual treatment because traditional curettes have the ability to alter and roughen the implant surface, it has been suggested that the tip material be softer than titanium. Cleaning using piezoelectric scalers or instruments can reduce bleeding on probing scores [6].
Laser therapy: Instrumentation produces equal results to erbium and carbon dioxide lasers in the surgical treatment of peri-implantitis, according to research.This is to minimise gingivitis and evaluate the healing process [17]. Erbium and chromium doped rays eradicate slime layer by up to 90% however unlike most mechanical therapies (Figure 4).Therapies with just an erbium beam generated substantially superior performance in terms of peri-implantitis haemorrhage when compared to mechanical approaches pocket depths, clinical attachment level, plaque index and gingival recessions all improved in both groups [6]. Goncalves et al. evaluated the level of reduction in bacteria of Enterococcus faecalis and Porphyromonas gingivalis contaminating three different implant surfaces after the use of a light beam of 980 nm diode and 1064 nm Neodymium doped Yttrium Aluminium Garnet (Nd:YAG) lasers. Prior to any procedure, non-surgical peri-implantitis therapy, such as treatment which uses shaft of light, may be used to reduce gingivitis and monitor the tissue regeneration.
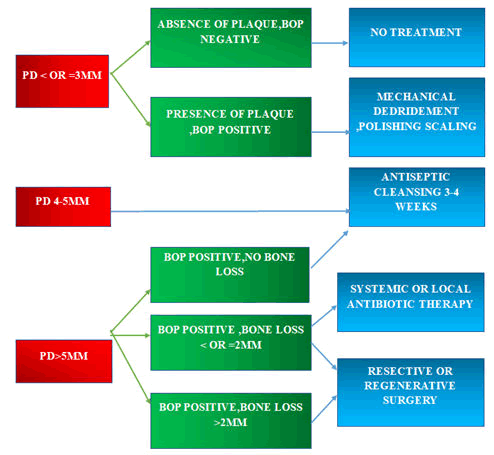
Figure 4: Cumulative Interceptive Supportive Therapy (CIST) Protocol.
Photodynamic therapy: In patients with moderate to severe chronic periodontitis, Photodynamic Treatment (PDT) with diode rays may provide a moderately preservative effect to scaling and root planning inside the application of an antibacterial PDT, light of a selected frequency coupled with a photo catalyst is employed to preferentially destroy aim microbes. In treating chronic periodontitis, neodymium doped and erbium doped YAG lasers may be identical to SRP in respect of probing penetration and gingivitis microbial activity, but not in terms of attachment gain [17]. Photodynamic therapy is now-a-days considered as a new treatment options.
Surgical interventions: Surgical treatment is reserved for the most severe peri-implantitis problems, which are often defined by pre-existing bone loss around the implant, as well as when non-surgical treatments have failed and the protocol says that they are used BOP is positive, PD is>than or likely to be 4 mm, and bone loss is larger than what would be expected during normal remodelling [5]. Surgical therapy incorporates the principles of non-surgical therapy as well as reconstructive and/or regenerative operations. “The peri-implantitis associated bone defects may be treated by reconstructive or resective procedures and sometimes the morphology of the osseous defect could be unsuitable for reconstructive techniques and so bone resection was performed.
Implantoplasty: Implantoplasty is a technique that smooths surfaces and lessens implant thickness, potentially jeopardising its implant's durability. Air-abrasive devices, brushes, laser, and chemical agents were all found to be less effective than this method for removal of debris [18].
By access flap surgery: Surfaces on implants have evolved throughout time, from machined titanium to a rough surface. Rough implant surfaces improve implant bone stability, but they can also promote plaque and bacterial colonisation, leading to peri-mucositis and peri-implantitis. The elimination of bacterial biofilm from the rough implant surface was thought to be a need for halting disease progression, Deppe et al.The use of locally or systemically administered antimicrobials, as well as mechanical (manual and ultrasonic scaler) or chemical debridement of the exposed implant surface, was all used in previous therapy regimens. Plaque removal and cleaning of the implant surface using a non-surgical debridement appears to be effective in the treatment of peri-mucositis, but only a minimal benefit has been documented in the treatment of peri-implantitis, Renvert et al.In addition to these treatments, many peri-implantitis therapy regimens have been documented in conjunction with implant surface cleansing. They include the replacement of the soft tissue flap-access flap with a hard tissue flap. Debridement, implant surface conditioning, and systemic and topical medications are all options. Antibiotic therapy, laser therapy, and reconstructive surgery. Serino and Turri looked examined the results of a surgical procedure for peri-implantitis that included pocket removal and bone re-contouring and was effective to do this. The majority of individuals and implants were treated for peri-implantitis.
By regenerative approaches: The degree of bone loss and subsequent form of the defect determine whether or not a surgical regenerative therapy should be pursued. After removing the granulation tissue and reflecting the flap, they are confirmed. Bone graft material, as well as a barrier membrane, if necessary. Regenerative therapy for peri-implantitis seeks to minimise PD, promote bone repair, and maintain peri-implant attachment and also minimise the mucosal recession [19]. Full regeneration and re-osseointegration are desired from a functional, aesthetic, and long-term survival standpoint. Allogenic and xenogenic grafts may be nearly interchangeable with autogenous tissue. The xenograft resulted in more radiographic bone fill and decreased pocket depths, although both treatments resulted to regenerate experimentally produced wounds in animal models using a variety of Graft materials and/or Resorbable membranes (GBR).
Use of bone graft: For such majority of the subjects, a postoperative idea relies on pockets clearance and bone re-contouring in between treatment was an appropriate cure for peri-implantitis and implant rupture. Systemic antibiotics combined with local surface debridement and granulation tissue removal resulted in significantly more “re-osseointegration " to an implant with a rough (SLA) surface than to an implant with a smooth (Turned) surface, according to research.Furthermore, the Er,Cr:YSGG laser has allowed for non-complicated regenerative osseous surgery around an implant.
Apart from surface cleansing, it appears that using a bone graft substitute to rectify anatomical situations for enhancing plaque control and lowering septic bacteria's hospitable environment and can be beneficial in crater produced defects. As a result, several types of bone graft materials have been used by different researchers to address peri-implantitis defects as a regenerative treatment.
Use of GTR therapy: Predictable treatment regimens for peri-implant infections that result in progressive osseointegration loss have yet to be established. Because the aetiology of peri-implant infections &advanced marginal periodontitis is so similar, same treatment approaches can be used in both cases. As a result, mechanical and chemical antibacterial techniques should be used to treat such lesions. Surface roughness of implants, on the other hand, may obstruct effective mechanical control of bacterial deposits. In addition, the best treatment for failed implants should include the regeneration of peri-implant tissues that have been lost. The principles of Guided Tissue Regeneration (GTR) have been developed in recent years to restore damaged periodontal tissues Nyman et al. GTR has also been used to successfully regenerate bone tissue in various types of jaw bone abnormalities and in conjunction with dental implants.
Following fatal photosensitization and directed bone regeneration, Shibli et al. assessed the healing ability and “re-osseointegration” of ligature-induced peri-implantitis defects next to various dental implant surfaces. The authors concluded that using “lethal photosensitization” in conjunction with GTR therapy to treat chronic peri-implantitis could result in considerable bone fill and re-osseointegration.
Use of Combination of “Bone Graft” and “Biomaterials”: Treatments for peri-implant bone loss have taken a variety of forms and there are still debates about which therapy is the most effective condition. Treatment with a closed approach and various combinations of medication. The use of mechanical debridement, topical antimicrobials and systemic antibiotics has proven to be effective. Although successful, there have been reports of failure. Several options exist prior to nonsurgical or surgical therapy, approaches have been utilised to clean the implant surface.All of them seek to get rid of bacteria while also creating a surface for re-osseointegration. Several studies have been conducted. The use of bone graft materials or a barrier membrane alone was studied. Graft of bone demineralized freeze dried materials is examples of materials used without a barrier membrane. Bovine inorganic bone, allogenic bone and hydroxyapatite As a result of this, although there was a positive clinical outcome, the number of patients studied was small and there were also malfunctions recorded. To induce bone regeneration and osseointegration, researchers have lately used bone graft material in combination with either GTR or biomaterials such as bone morphogenetic proteins, growth factors and emdogain [20].
Resective therapy: Periodontitis and resective surgeries reduce BOP, probing depths and clinical indicators of inflammation. Ostectomy and osteoplasty to remove the preimplant osseous defect, as well as bacterial decontamination, are the main principles. In a 3 years radiographic evaluation, Romeo et al. discovered that marginal bone loss after resective surgery with implantoplasty was substantially lower than after resective therapy alone. Ostectomy and osteoplasty in combination with implantoplasty are effective therapies for reducing or preventing peri-implantitis progression [6].
Reconstructive procedures: During peri-implantitis the loss which occurs leads to varying morphologies with osseous defects.A buccal and lingual bone wall may remain when the ridge's breadth exceeds that of the peri-implantitis lesion and a crater may occur. During the progression of peri-implantitis, the buccal and lingual bone walls will be resorbed and lost in places with a small ridge. As a result, peri-implantitis sites frequently have an angular bone defect solely on the mesial and distal aspects of the implant (Table 1). A reconstructive technique may be considered in patients with peri-implantitis presenting with circumferential bone craters [21].
| Methods of decontamination | Advantages | Disadvantages |
|---|---|---|
| Stainless steel curettes | Ideal removal of debris. | Surface variation of implant is significant |
| Titanium Curettes | Ideal removal of debris. | Surface variation of implant is minimal |
| Plastic Curettes | Surface alteration of implant is not there. | Fragile |
| Ultrasonic with dedicated tip | Debris removal is excellent. | Minimal modification in implant surface |
| Ultrasonic without dedicated tip | There is total withdrawal of debris. | Surface variation is appropriate |
| Brushes made up of titanium | Fine removal of debris. | Noticeable implant surface variation and delicate |
| Air- abrasive devices | Debris withdrawal is excellent and no alteration in surface. | Unnecessary application induces soft tissue injury. |
| Lasers | Toxic contamination is excellent. | Validate relying upon dosage |
| Chlorhexidine | None | No extra effects |
| Chemical agents (H2O2,H3PO4,EDTA,etc.) | Provocative | Rusting with pH of <3 and morphologic agents. |
| Systemic and local antibiotics. | Limited evidences. |
Table 1: Advantages and disadvantages of decontamination.
Discussion
Peri-implantitis is arguably one of the most significant risk factors associated with late implant failures. Several treatment regimens have been described in conjuction with surface decontamination which includes the elimination of soft tissue flap access flap debridement, implant surface conditioning, use of laser therapy and respective surgery. Various authors have used regenerative materials like bone graft, GTR, combination of both for the treatment of peri-implantitis. Various authors had applied nanotechnology for implant therapy to promote osseointegration as well as for the treatment of peri-implantitis and also stated that future management of peri-implantitis can be directed towards the use of nanotechnology.
Conclusion
As the frequency of peri-implantitis continues of increase, the treatment of peri-implantitis has become the daily dental practice. It's crucial to underset and the variations between the gingiva around natural teeth and the mucosa around dental implants, as well as the environmental conditions in the supragingival, subgingival and periapical areas, in order to recognise and diagnose the disease. In peri-implantitis, the microbiota is more diversified, with gram-negative bacteria accounting for a high fraction of the microorganisms. Periodontal care is required every three months for dental implants. Considering the bacterial repopulation's timeframe, this might take months. After the condition has been identified; therapy should begin as soon as possible.
References
- Mistry RA, Pisulkar SK, Godbole S, et al. An appraisal of bone resorption in completely edentulous diabetic and nondiabetic patients at prospective implant site in anterior mandible using digital volumetric tomography and its correlation with glycemic control: A case–control study. Natl J Maxillofac Surg 2020; 11:28.
- Jain P, Jain M, Gaikwad RN, et al. Role of inflammation and inflammatory biomarkers in dental implant procedures: A comprehensive review. J Datta Meghe Inst Med Sci Univ 2020; 15:715.
- Khungar PN, Dahane TM, Revankar RP, et al. Customized Treatment Option for Malpositioned Dental Implant Placed in Aesthetic Zone. J Evol Med Dent Sci 2020; 9:2930-2935.
- Singh A, Sheok V, Bhardwaj A, et al. Dental implant design-an insight overview. J Med Pharm Allied Sci 2015; 10:3101–3105.
- Robertson K, Shahbazian T, MacLeod S, et al. Treatment of peri-implantitis and the failing implant. Int J Mol Sci 2018; 19:3157.
- Smeets R, Henningsen A, Jung O, et al. Definition, etiology, prevention and treatment of peri-implantitis–a review. Head Face Med 2014; 10:1-3.
- Hwang G, Blatz MB, Wolff MS, et al. Diagnosis of Biofilm-Associated Peri-Implant Disease Using a Fluorescence-Based Approach. Dentistry J 2021; 9:24.
- Schwarz F, Derks J, Monje A, et al. Peri-implantitis. J Clin Periodontol 2018; 45:246-266.
- Charde P, Thakare KS, Bhongade ML, et al. Reconstruction of Interim plant Papilla by Demineralized Freeze-dried Bone Allograft Block Fixed by Titanium Screw in Maxillary Esthetic Zone. J Contemp Dent Pract 2020; 21:1205-1209.
- Jalaluddin M, Ahamed S, Javali MA, et al. Peri-implantitis - a birdâ??s eye view. Int J Health Sci Res 2013; 3:112-120.
- Radaelli K, Alberti A, Corbella S, et al. The Impact of Peri-Implantitis on Systemic Diseases and Conditions: A Review of the Literature. Int J Dent 2021; 2021.
- Fragkioudakis I, Tseleki G, Doufexi AE, et al. Current Concepts on the Pathogenesis of Peri-implantitis: A Narrative Review. Eur J Dent 2021; 5:379–387.
- Soler MD, Hsu SM, Fares C, et al. Titanium Corrosion in Peri-Implantitis. Mater. 2020; 13:5488.
- Renvert S, Persson GR, Pirih FQ, et al. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J Clin Periodontol 2018; 45:278-285.
- Kormas I, Pedercini C, Pedercini A, et al. Peri-implant diseases: Diagnosis, clinical, histological, microbiological characteristics &treatment strategies. A narrative Review. Antibiotics. 2020; 9:835.
- Saito H, Kensara A, Masri R, et al. Current Concepts on the Etiology and Development of Peri-Implantitis: what should you know? JCosmet Dent. 2021; 36.
- Alassy H, Pizarek JA, Kormas I, et al. Antimicrobial adjuncts in the management of periodontal and peri-implant diseases and conditions: a narrative review. 2021; 3.
- Kormas I, Pedercini C, Pedercini A, et al. Peri-implant diseases: Diagnosis, clinical, histological, microbiological characteristics and treatment strategies. A narrative Review. 2020; 9:835.
- Barootchi S, Wang HL. Peri-implant diseases: Current understanding and management. Int J Oral Implants 2021; 14:263-282.
- Nayak DG, Uppoor A, Mahesh CP, et al. Textbook of Periodontology and Oral Implantology-E-Book. Elsevier 2014.
- Lindhe J, Karring T, Lang NP, et al. Clinical periodontology and implant dentistry. Blackwell 2003.
Author Info
Tanishka Taori*, Bhairavi Kale and Khushboo Durge
Department of Periodontics, Sharad Pawar Dental College and Hospital, Datta Meghe Institute of Medical Sciences DMIMS (Deemed to be University) Sawangi (Meghe): Wardha 442001, Maharashtra, IndiaReceived: 27-Jun-2022, Manuscript No. JRMDS-22-47350; , Pre QC No. JRMDS-22-47350; Editor assigned: 29-Jun-2022, Pre QC No. JRMDS-22-47350; Reviewed: 13-Jul-2022, QC No. JRMDS-22-47350; Revised: 26-Aug-2022, Manuscript No. JRMDS-22-47350; Published: 05-Sep-2022