Review - (2021) Volume 9, Issue 3
Periodontitis, the Current Cellular and Molecular Histopathologic Representation: A Narrative Review
*Correspondence: Aljoharah A Alsinaidi, Department of Periodontics and Community Dentistry, Collage of Dentistry, King Saud University, Riyadh, Saudi Arabia, Email:
Abstract
Periodontal disease is an inflammatory condition, clinically characterized by loss of periodontal attachment and bone loss around the tooth. This inflammatory process is initiated as a result of the pathogenic microbial insult which leads to an intense immunoinflammatory infiltrate in the periodontal tissue. This might lead to tooth loss if not appropriately managed. In gingivitis, a reversible form of periodontal disease that does not result in bone loss. Roy Page and Hubert Schroeder reported the first systematic model describing the host response in four types of histopathologic lesions: “initial,” “early, “established” and “advanced”. Although understanding of the periodontal pathogeneses in the development of gingivitis and periodontitis is largely based on the microbial involvement, further studies highlighted the complexity of the interactions between the periodontopathogens, host response, and modifying factors that contribute to the ultimate outcome of disease development and progression. The aim of this paper is to review the current aspects in the cellular and molecular pathogenesis of periodontal diseases.
Keywords
Periodontitis, Pathogenesis, Immune, T-cells, NK-cells
Introduction
Periodontal diseases are the most common oral bacterial infection worldwide [1]. They occur as a result of a complex interaction between the periodontopathogens and host's inflammatory and immune system in addition to an interplay of environmental and genetic factors [2]. Periodontal diseases include gingivitis that is a reversible inflammation confined to the gingival tissues, and periodontitis, an irreversible and destructive form [3].
Methodology
The Pub-Med database of the US National Library of Medicine was searched using the keywords: “periodontal” and “pathogenesis”. Google Scholar database was also searched for the additional literature search. The papers were manually selected after reading the abstracts and only relevant papers were included only if they were: (i) Written in English language, (ii) Published in the international peer-reviewed journals. Citation tracking was completed for included studies using Endnote™, version 9 (Clarivate Analytics, Boston, MA, USA).
The classic histopathologic characteristic of periodontal disease
sThe classical histopathologic ‘road- map’ of periodontal disease was described by Page et al. (Figure 1), based on assessment of tissues of naturally occurring and experimentally induced gingivitis and periodontitis in humans and animals and four lesions were reported. The initial, early and established lesions are recognized as distinctive stages of gingivitis, and the advanced lesion is distinguished as apparent periodontitis. In the initial lesion, the gingival tissues respond to inflammation within 2–4 days from plaque accumulation as an acute exudative vasculitis in the plexus of venules lateral to the junctional epithelium. In addition, polymorphonuclear leukocytes (PMNs) migrate from the junctional epithelium to the gingival sulcus and the perivascular collagen is lost. Within 4–10 days, the early lesion develops and shows dense infiltrate of T lymphocytes and other mononuclear cells, and alteration in the gingival fibroblasts. The established lesion occurs within 2–3 weeks after plaque accumulation. It is dominated by B lymphocytes (plasma cells) and reveals more loss of gingival connective tissue matrix. A gradual gingival pocket is established during this stage but no bone loss is visible. Clinically, the established lesion demonstrates moderate to severe gingivitis which may progress to the advanced lesion. In the advanced lesion, the inflammatory infiltrate is composed of plasma cells and macrophages and it is associated with pocket formation and alveolar bone resorption [4-6].
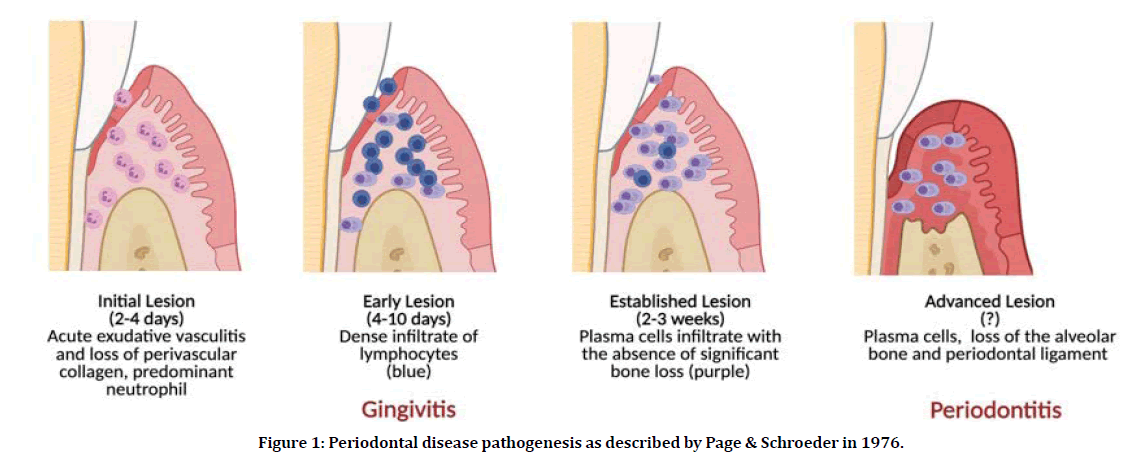
Figure 1: Periodontal disease pathogenesis as described by Page & Schroeder in 1976.
Although understanding of the periodontal pathogeneses in the development of gingivitis and periodontitis is largely based on the microbial involvement, further studies highlighted the complexity of the interactions between the periodontopathogens, host response, and modifying factors that contribute to the ultimate outcome of disease development and progression [5,6].
Identifying the cellular and molecular hallmarks
Based on to the most well-established hypothesis of pathogenesis, host response plays a significant role in the complex etiology of periodontal disease [6]. In periodontitis, the host response is stimulated by the microbial challenges to initiate an inflammatory process as a defense mechanism. If not treated, the inflammation will progress to cause gingival connective tissue destruction and alveolar bone resorption. Environmental, habitual and genetic factors may change the pathways of the disease progression to either control the disease so the patient will be less susceptible to periodontitis or exacerbate the reaction making the patient more susceptible to periodontitis. This may explain the enormous differences in the patterns of destruction and patients' susceptibilities to periodontitis [7].
Pathogen virulence
Periodontal disease occurs when the equilibrium between the bacterial virulence and host’s defense is disturbed resulting to a pathological response in the host’s tissues [8]. The bacterial virulence can be classified into three categories; (i) Bacterial capability to adhere and colonize the area; (ii) Bacterial invasion to the connective tissue and; (iii) Interference with the host’s defense mechanism.
Adhesion and colonization
The balance between the bacterial colonization and host’s response determines the microbial homeostasis in the oral cavity. It starts at birth and continues throughout life [9,10]. If this balance is interrupted, the periodontopathogens will overcome the existing microbial homeostasis and cause pathology [11]. The gingival pocket provides an ideal environment for the anaerobic, motile, and low adherent periodontal pathogenic bacteria including Prophromonas gingivalis (P. gingivalis), Tannerella forsythia (T. forsythia), Treponema denticola (T. denticola), Fusobacterium species, Prevotella species, Campylobacter species, and anaerobic streptococci [12]. Furthermore, the gingival pocket offers the periodontal pathogenic bacteria a mechanical persistence and nutrients in the gingival exudate such as hemin, vitamins and hormones [11]. Concurrently, these bacteria produce numerous virulent factors that may interfere with the host’s immune system such as proteolytic enzymes and endotoxins/lipopolysaccharides (LPS) and consequently they proliferate sub-gingivally to initiate and/or maintain inflammatory process [11,12].
Cell and tissue invasion
Bacterial invasion to the connective tissue is a critical event in the development of periodontitis [13,14]. It takes place if the junctional epithelium is disrupted with ulceration. Many acute infections had demonstrated bacterial invasion associated with neutrophil attraction. For example, in acute necrotizing ulcerative gingivitis, spirochetes are known to invade the connective tissues [15,16]. Periodontal abscess is also associated with bacterial invasion and growth into the tissues leading to fistula formation. In chronic periodontal disease, it is still controversial whether bacterial invasion takes place or not. However, it is confirmed that Aggregatibacter actinomycetemcomitans (A. actinomycetemcomitans) invade the underlying connective tissues in the aggressive form of periodontitis [14-17].
Bacterial invasion of periodontal tissues occurs through either intercellular or intracellular routes [14-18]. The interruption of the junctional epithelium allows motile bacteria such as Spirochetes, Treponema and Campylobacter species to penetrate the underlying connective tissues. On the other hand, some pathogenic bacteria such as Treponema species and P. gingivalis have the ability to produce specific gingipains (E-cadherin and occludin) that degrade proteins of the junctional epithelium [19]. The intracellular route attracts more attention as P. gingivalis T. forsythia, P. intermedia, and C. rectus were observed within the cells of periodontal tissue [20-22].
Evading and modulating of the host’s response
The host reacts to the bacterial insult through inflammation and recruiting inflammatory mediators, neutrophils, macrophages, and antibodies [23]. In most cases, the neutrophils and macrophages can phagocytose the periodontal pathogenic bacteria. However, some bacteria have a specific ability to escape phagocytosis by producing leukotoxin or forming capsule and may result to spread of inflammation [24,25]. Leukotoxin is a well-known virulent factor produced by A. actinomycetemcomitans [26,27]. Furthermore, periodontopathogens can modulate the host immune response by producing endotoxins/lipopolysaccharides (LPS) that interact with B and T lymphocytes [28]. The toxicity of LPS varies greatly among various bacteria like P. gingivalis [29]. The outer membrane vesicles formed by P. gingivalis are mainly LPS membrane structures that contribute to P. gingivalis host interaction and pathogenicity [30].
T - Cells and periodontitis
When periodontitis is not resolved, a dense inflammatory infiltrate in the connective tissue activates the host immune response that is mainly mediated by the inflammatory cells such as neutrophils, monocytes/macrophages, and T and B lymphocytes [31]. T-lymphocytes are immune cells that are involved in the host defense and control immune-mediated inflammatory disease development. They can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on the cell surface. They are subdivided into Th, Treg, T-cytotoxic (CD8+), natural killer, and memory cells. In response to bacterial insult, antigen-presenting cells (APCs) will be activated and trigger the naive T-helper cells (Th0) to differentiate into T- cells subsets such as Th1, Th2, Th9, Th17, T-follicular helper (Tfh), and regulatory T-cells (Treg) based on their unique cytokine properties [31].
The role of T cells in periodontitis has been extensively revised by Campbell et al. The triggering of pro-inflammatory cytokines, such as IL-1β, IL-17E (IL-25) and IL-17 as a result of activation of Th1, Th2, Th17 and other immune cells such as dendritic cells, neutrophils, and B cells will result into expression of the receptor activator of nuclear factor kappa-B-ligand (RANKL) causing alveolar bone resorption [31].
The association of Th1 and Th2 with periodontal disease has been reported in the literature. A number of studies observed decreased levels of Th1 in the gingival crevicular fluid (GCF) [32], gingival mononuclear cells at periodontitis sites [33] and peripheral blood mononuclear cells in patients with periodontitis cocultured with P. gingivalis and F. nucleatum [34]. However, increased Th2 responses in periodontitis patients have been detected in gingival tissues [35,36], extracted gingival mononuclear cells [37] and GCF [38]. In addition, both Th1 and Th2 cytokines have been observed in experimental studies of cells extracted from the periodontal lesions [39,40] and IL-1β, TNF-α, and IL-17 were highly expressed in patients with periodontitis [41].
T cells have a critical role in the secretion of IL- 17 which is associated with alveolar bone loss. In patients with chronic periodontitis, levels of IFN-γ, IL-17A, and T-bet mRNA were significantly higher than healthy controls [42]. This might suggest that Th1and Th17 cells play a significant role in the pathogenesis of chronic periodontitis. The major source of IL-17 is CD4+ T cells, which increased significantly in periodontitis patients [43,44]. Furthermore, Th17 cells in the presence of periodontitis are dependent on the local pathogenic bacteria, and both IL-6 and IL-23 are needed for their accumulation [45]. Thus, the immunoregulatory control of T cells and their effector cytokines is fundamental in defining the ultimate outcome of chronic periodontitis.
Natural killer cells and periodontitis
Natural Killer cells (NK cells) are discrete cytotoxic lineage of lymphocytes which play an important role in the pathobiology of periodontitis. NK cells are one of the major components of the innate immune system. They play a regulatory function by secreting cytokines and gamma interferon (IFN-γ) [46,47]. NK cells are activated by CD2-like receptors which were reported to activate cytotoxic cells which in turn activate CD8 in T and B cells [48-50]. This activation process might be involved in periodontal tissue destruction [50-52]. Furthermore, production of IFN-γ by NK cells has been observed to occur when dendritic cells (DCs) were cocultured with periodontopathogens such as P. gingivalis and A. actinomycetemcomitans [53,54]. Moreover, F. nucleatum can activate receptor NKp46 in NK cells leading to periodontal bone loss [55]. Recently, Gaudilliere and his coworkers [56] observed exaggerated proinflammatory responses to P. gingivalis–derived lipopolysaccharides in the circulating neutrophils and monocytes of patients with chronic periodontitis compared to healthy controls. This immune alteration was not detectable three weeks after periodontal treatment. Although, the role of NK cells in periodontal disease is well established, the mechanisms underlying their activation during periodontal disease are not fully understood [57].
Conclusion
Although the proposed model is reasonable, our understanding of how inflammatory host response is regulated is far from complete. New discoveries are needed to enhance the information obtained from the currently available indicators and understand the disease mechanisms. As a result, there is a great increase in the interest to apply emerging technologies in order to improve the understanding of the disease processes and the underlaying factors.
References
- Hernández M, Vernal R, Sorsa T, et al. The role of immuno-inflammatory response in the pathogenesis of chronic periodontitis and development of chair-side point of care diagnostics. In Nurcan Buduneli (Ed.). Pathogenesis and treatment of periodontitis In Tech Publishing Co Chapter 3, 2012.
- Teles F, Wang Y, Hajishengallis G, et al. Impact of systemic factors in shaping the periodontal microbiome. Periodontol 2021; 85:126–160.
- Caton JG, Armitage G, Berglundh T, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions-introduction and key changes from the 1999 classification. J Periodontol 2018; 89:1.
- Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Laboratory investigation. J Tech Methods Pathol 1976; 34:235-249.
- Page RC, Davies P, Allison AC. Effects of dental plaque on the production and release of lysosomal hydrolases by macrophages in culture. Arch Oral Biol 1973; 18:1481–1495.
- Bostanci N, Belibasakis GN. Periodontal pathogenesis: Definitions and historical perspectives. InPathogenesis of periodontal diseases 2018; 1-7.
- Hajishengallis G, Korostoff, JM. Revisiting the page & schroeder model: the good, the bad and the unknowns in the periodontal host response 40 years later. Periodontol 2017; 75:116-151.
- Nędzi-Góra M, Kowalski J, Górska R. The immune response in periodontal tissues. Arch Immunol Therap Exp 2017; 65:421-429.
- Darveau RP. Periodontitis: A polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010; 8:481–890.
- Marsh PD, Moter A, Devine DA. Dental plaque biofilms: Communities, conflicts and control. Periodontol 2011; 55:16–35.
- Marsh PD. The commensal microbiota and the development of human disease–an introduction. J Oral Microbiol 2015; 7:e29128.
- Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Periodontol 2011; 38:28–35.
- Socransky SS, Haffajee AD, Cugini MA, et al. Microbial complexes in subgingival plaque. J Clin Periodontol 1998; 25:134–144.
- Allenspach-Petrzilka GE, Guggenheim B. Bacterial invasion of the periodontium: an important factor in the pathogenesis of periondontitis? J Clin Periodontol 1983; 10:609–617.
- Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2010; 52:68–83.
- Listgarten MA. Electron microscopic observations on the bacterail flora of acute nectrotizing ulcerative gingivitis. J Periodontol 1965; 36:328–339.
- Listgarten MA. Structure of the microbial flora associated with periodontal health and disease in man. A light and electron microscopic study. J Periodontol 1976; 47:1–18.
- Ji S, Choi YS, Choi Y. Bacteril invasion and persistence: critical events in the pathogenesis of periodontitis? J Periodontol Res 2015; 50:570–585.
- Lux R, Miller JN, Perk NH, et al. Motility and chemotaxis in tissue penetration of oral epithelial cell layers by Treponema denticola. Infect Immun 2001; 69:6276–6283.
- Katz J, Yang QB, Zhang P, et al. Hydrolysis of epithelial junctional proteins by Porphyromonas gingivalis gingipains. Infect Immun 2002; 70:2512–2518.
- Rudney JD, Chen R, Zhang G. Streptococci dominate the diverse flora within buccal cells. J Dent Res 2005; 84:1185–1171.
- Rudney JD, Chen R, Sedgewick GI. Actinobacillus actinomycetemcomitans, Porphyromonas gongivalis and Tannerella forsythensis are components of a polymicrobial flora within human buccal cells. J Dent Res 2005; 84:59–63.
- Dibart S, Skobe Z, Snapp KR, et al. Identification of bacterial species or in crevicular epithelial cells from healthy and periododntitis. Oral Microbiol Immunol 1998; 13:30–35.
- Hajishengallis G, Chavakis T, Lambris JD. Current understanding of periodontal disease pathogenesis and targets for host‐modulation therapy. Periodontol 2020; 84:14‐ 34.
- Johansson A, Sandström G, Claesson R, et al. Anaerobic neutrophil-dependent killing of Actinobacillus actinomycetemcomitans in relation to the bacterial leukotoxicity. Eur J Oral Sci 2000; 108:136–146.
- Singh A. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response evasion of phagoscytosis and increase in virulence. Infect Immun 2011; 79:4533–4542.
- Johansson A. Aggregatibacter actinomycetemcomitans leukotoxin: A powerful tool with capacity to cause imbalance in the host inflammatory response. Toxins 2011; 3:242–359.
- Kachlany SC. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res 2010; 89:561–570.
- Peterson JW. Chapter 7: Bacterial pathogenesis. In: Baron S. Medical microbiology. 4th ed. Galveston, TX: University of Texas Medical Branch 1996.
- Paramonov N, Aduse-Opoku J, Hashim A, et al. Identification of the link- age between A-polysaccharide and the core in the A-lipopolysaccharide of Porphyromonas gingivalis W50. J Bacteriol 2015; 197:1735–1746.
- Xie H. Biogenesis and function of Porphyromonas gingivalis outer membrane vesicles. Future Microbiol 2015; 10:1517–1527.
- Campbell L, Millhouse E, Malcolm J, et al. T cells, teeth and tissue destruction—what do T cells do in periodontal disease? Mol Oral Microbiol 2016; 31:445–456.
- Pilon M, Williams‐Miller C, Cox DS. Interleukin‐2 levels in gingival crevicular fluid in periodontitis. J Dent Res 1991: 70:550.
- Fujihashi K, Kono Y, Yamamoto M, et al. Interleukin production by gingival mononuclear cells isolated from adult periodontitis patients. Dent Res 1991; 70:550.
- Gemmell E, Seymour GJ. Modulation of immune responses to periodontal bacteria. Curr Opin Periodontol 1994: 94:28–38.
- Tokoro Y, Matsuki Y, Yamamoto T, et al. Relevance of local Th2‐type cytokine mRNA expression in immunocompetent infiltrates in inflamed gingival tissue to periodontal diseases. Clin Exp Immunol 1997; 107:166– 174.
- Yamazaki K, Nakajima T, Gemmell E, et al. IL‐4‐ and IL‐6‐producing cells in human periodontal disease tissue. J Oral Pathol Med 1994; 23:347–353.
- Manhart SS, Reinhardt RA, Payne JB, et al. Gingival cell IL‐2 and IL‐4 in early‐onset periodontitis. J Periodontol 1994: 65:807–813.
- Reinhardt RA, McDonald TL, Bolton RW, et al. IgG subclasses in gingival crevicular fluid from active versus stable periodontal sites. J Periodontol 1989; 60:44– 50.
- Fujihashi K, Yamamoto M, McGhee JR, et al. Type 1/type 2 cytokine production by CD4+T cells in adult periodontitis. J Dent Res 1994; 73:204.
- Prabhu A, Michalowicz BS, Mathur A. Detection of local and systemic cytokines in adult periodontitis. J Periodontol 1996; 67:515–522.
- Arzu B, Ainola M, Hukkanen M, et al. Konttinen. "MMPs, IL-1, and TNF are regulated by IL-17 in periodontitis. J Dent Res 2007; 86:347-351.
- Chen ML, Sundrud MS. Cytokine networks and T-cell subsets in inflammatory bowel diseases. Inflamm Bowel Dis 2016; 22:1157–1167.
- Dutzan N, Konkel JE, Greenwell-Wild T, et al. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol 2016; 9:1163–1172
- Dutzan N, Abusleme L, Konkel JE, et al. Isolation, characterization and functional examination of the gingival immune cell network. J Vis Exp 2016; 108:53736.
- Dutzan NKT, Abusleme L, Greenwell-Wild T, et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med 2018; 10:eaat0797
- Caligiuri MA. Human natural killer cells. Blood 2008; 112:461–469.
- Chaerita M, Masulili LC, Auerkari EI. "Interferon-gamma+ 874A/T polymorphism in relation to susceptibility of periodontal disease: Systematic review. In AIP Conference Proceedings 2092: 040023.
- ClausM, MeinkeS, Bhat R, et al. Regulation of NK cellactivity by 2B4, NTB-A and CRACC. Front Biosci 2008; 13:956 –965.
- Cruz-Munoz ME, Dong Z, Shi X, et al. Influence of CRACC, a SLAM family receptor coupled to the adaptor EAT-2, on natural killer cell function. Nat Immunol 2009; 10:297–305.
- Figueredo CM, Lira R, Love RM. T and B cells in periodontal disease: New functions in a complex scenario. Int J Mol Sci 2019; 20:3949.
- Wilensky A, Chaushu S, Shapira L. The role of natural killer cells in periodontitis. Periodontol 2015; 69:128-141.
- Kindstedt E, Koskinen Holm C, Palmqvist P, et al. Innate lymphoid cells are present in gingivitis and periodontitis. J Periodontol 2019; 90:200-207.
- Kikuchi T, Willis DL, Liu M, et al. Dendritic-NK cell interactions in P. gingivalis specific responses. J Dent Res 2005; 84:858–862.
- Kikuchi T, Hahn CL, Tanaka S, et al. Dendritic cells stimulated with Actinobacillus actinomycetemcomitans elicit rapid gammainterferon responses by natural killer cells. Infect Immun 2004; 72:5089 –5096.
- Chaushu S, Wilensky A, Gur C, et. al. 2012. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog 2012; 8:e1002601.
- Gaudilliere DK, Culos A, Djebali K, et al. Systemic immunologic consequences of chronic periodontitis. J Dent Res 2019; 98: 985-993.
Author Info
Department of Periodontics and Community Dentistry, Collage of Dentistry, King Saud University, Riyadh, Saudi ArabiaCitation: Aljoharah A Alsinaidi, Periodontitis, The Current Cellular and Molecular Histopathologic Representation: A Narrative Review, J Res Med Dent Sci, 2021, 9 (3):126-131.
Received: 17-Feb-2021 Accepted: 18-Mar-2021 Published: 25-Mar-2021
