Research - (2022) Volume 10, Issue 7
Pilot Study on the Therapeutic Effects of Cisplatin and Docetaxel Superselective Intra-Arterial Infusion and Systemic 5-Fluorouracil Combination Chemotherapy for Stage II Squamous Cell Carcinoma of the Tongue with Greater than 4 mm Depth of Invasion
Shigeo Tanaka1*, Maya Oshima1, Hideo Niwa2, Yasuhide Makiyama2, Kayo kuyama3, Hirayama4 and Masamichi Komiya1
*Correspondence: Shigeo Tanaka, Department of Oral Surgery, Nihon University School of Dentistry at Matsudo, Matsudo Chiba 271-8587, Japan, Email:
Abstract
This study aimed to investigate the effects of superselective intra-arterial infusion of cisplatin and docetaxel and systemically administered 5-fluorouracil combination chemotherapy (i.e., TPF chemotherapy) in patients with stage II squamous cell carcinoma of the tongue with greater than 4 mm depth of invasion (DOI). Eight patients diagnosed with stage II squamous cell carcinoma of the tongue with DOI >4 mm between December 2007 and July 2017 who underwent TPF chemotherapy at Nihon University Hospital at Matsudo were examined retrospectively. Six of the eight patients were managed under wait-and-see policy after chemotherapy and two patients underwent surgery for the primary lesion after chemotherapy. The disease-specific survival (DFS) and recurrence-free survival (RFS) rates of the six patients managed under the wait-and-see policy were compared using the Kaplan–Meier method, and delayed lymph node metastasis was monitored. Both the response and complete response rates were 100% in all patients. The median follow-up period of the six patients was 2915 days. The 5-year DFS and RFS (organ-preserving) rates were 83.3%. The median follow-up period for the two patients who underwent surgery was 2614 days, and primary lesion recurrence was not observed. Delayed metastasis to the cervical lymph nodes was not observed in any of the eight patients. No severe adverse events related to chemotherapy were noted. This study demonstrated the high therapeutic potential of the TPF chemotherapy for stage II squamous cell carcinoma of the tongue with DOI >4 mm.
Keywords
Tongue squamous cell carcinoma, Depth of invasion, Cervical lymph node metastasis, Superselective intra- arterial chemotherapy, TPF chemotherapy
Introduction
Surgery is considered the standard therapy for stage I (T1N0M0) and II (T2N0M0) tongue cancer [1]. However, many studies report the poor prognosis of patients with squamous cell carcinoma of the tongue with a depth of invasion (DOI) >4 mm, regardless of the cancer stage [2–5]. Therefore, even early-stage cases with DOI >4 mm require aggressive treatment, such as multimodal therapy [2–6]. Combined chemotherapy consisting of a superselective intra-arterial infusion of cisplatin (CDDP) and docetaxel (DTX) and systemically administered fluorouracil (5-FU) (i.e., TPF chemotherapy) has been administered at the Nihon University Hospital at Matsudo as induction chemotherapy to prevent the recurrence of the primary lesion in patients with stage II squamous cell carcinomas of the tongue with DOI >4 mm. Furthermore, we adopted a wait-and-see policy for patients who achieved a complete response (CR) after induction chemotherapy as per clinical and imaging findings, strongly desired to preserve their tongue and refused surgery, provided a rigorous follow-up is conducted.
This retrospective pilot study aimed to investigate the effects of TPF chemotherapy in patients with stage II squamous cell carcinomas of the tongue with DOI >4 mm.
Subjects and Methods
The subjects were eight patients who were diagnosed with stage II (T2N0M0) squamous cell carcinoma of the tongue with DOI >4 mm between December 2007 and September 2017 at the Department of Oral Surgery, Nihon University Hospital at Matsudo and underwent TPF chemotherapy (Table 1). Cancer staging was performed according to the Union for International Cancer Control (UICC) classification [7] and confirmed via contrast-enhanced magnetic resonance imaging (MRI). This study was an open study; the Ethics Committee of Nihon University School of Dentistry at Matsudo approved this study to be conducted by posting an information disclosure document on the website of Nihon University School of Dentistry at Matsudo instead of individual consent forms. This study was approved by the Ethics Review Board of Nihon University School of Dentistry at Matsudo (approval no.: EC 20-012).
CDDP + DTX Superselective intra-arterial chemotherapy was administered after inserting a micro catheter into the radial artery using the Seldinger method to identify the tumor-feeding artery. Digital subtraction angiography was performed, and indocyanine green was used to stain the tissues for identifying the tumor-feeding arteries (Figure 1A and 1B). After identifying the tumor-feeding arteries, sodium thiosulfate (STS) was administered via the central venous catheter to neutralize CDDP. After initiating STS administration, antineoplastic agents, DTX at 20–30 mg/m2 and CDDP at 100–150 mg/m2, were administered to the tumor-feeding arteries super selectively. Next, 5-FU (500–750 mg/m2/day) was systemically administered for 3 days starting from the day after administering the Superselective intraarterial chemotherapy. Two to four cycles of TPF chemotherapy were administered at 3- to 4-week intervals.
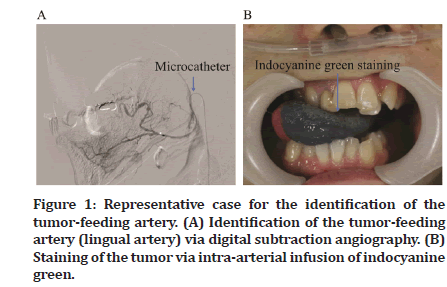
Figure 1: Representative case for the identification of the tumor-feeding artery. (A) Identification of the tumor-feeding artery (lingual artery) via digital subtraction angiography. (B) Staining of the tumor via intra-arterial infusion of indocyanine green.
Antitumor effects were evaluated using clinical, imaging (computed tomography, MRI, positron emission tomography), and histopathological findings and graded using the revised Response Evaluation Criteria in Solid Tumours (RECIST) guidelines (version 1.1) [8]. Adverse events associated with chemotherapy were assessed using the Common Terminology Criteria for Adverse Events Version 4.0 [9]. The prognosis was analyzed separately for the six patients who were managed under the wait-and-see policy after chemotherapy and two patients who underwent surgery for the primary lesion after chemotherapy. Statistical analysis was performed using the Kaplan–Meier method to compare the disease-specific survival (DSS) and recurrence-free survival (RFS) rates of the six wait-and-see patients. The occurrence of delayed cervical lymph node metastasis was investigated in all patients.
Results
Patient age and sex
The subjects were of age 27–75 years (median, 57 years) and consisted of seven men and one woman (Tables 1 and Table 2).
| Gender | Age | Maximum diameter of the tumor (mm) | Tumor depth (mm) | Antitumor effects | Surgery for the primary lesion | Recurrence of the primary lesion | Delayed cervical lymph node metastasis | Follow-up period (days) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Female | 39 | 22 | 7.5 | CR | Yes | No | No | 1482 |
| 2 | Male | 50 | 25 | 9 | CR | No | No | No | 2604 |
| 3 | Male | 75 | 22 | 6 | CR | No | No | No | 2707 |
| 4 | Male | 61 | 29 | 5 | CR | No | No | No | 3122 |
| 5 | Male | 27 | 21 | 9 | CR | No | No | No | 3587 |
| 6 | Male | 74 | 23 | 9 | CR | No | No | No | 4334 |
| 7 | Male | 53 | 26 | 6 | CR | Yes | No | No | 3745 |
| 8 | Male | 66 | 29 | 9 | CR | No | Yes | No | 572 |
| CR: Complete response | |||||||||
Table 1: Characteristics of the patients with squamous cell epithelial cancer of the tongue who underwent treatment with superselective intra-arterial infusion of cisplatin and docetaxel and systemically administered 5-fluorouracil.
Antitumor effects and adverse events
Antitumor effects
After the completion of chemotherapy, antitumor effects were confirmed relatively early in all patients (Figure 2). A reduction in tumor size was observed on imaging (Figure 3). The response and CR rates were both 100% for all patients (Table 2).
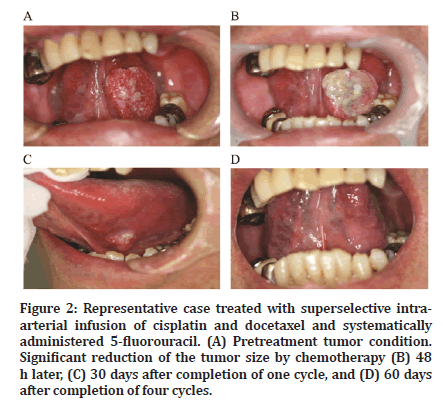
Figure 2:Representative case treated with superselective intraarterial infusion of cisplatin and docetaxel and systematically administered 5-fluorouracil. (A) Pretreatment tumor condition. Significant reduction of the tumor size by chemotherapy (B) 48 h later, (C) 30 days after completion of one cycle, and (D) 60 days after completion of four cycles.
| Case Summary | Median age | Response rate (%) | Complete response rate (%) | Delayed cervical lymph node metastasis rate (%) | Median follow-up period (days) |
|---|---|---|---|---|---|
| All cases | 57 | 100 | 100 | 0 | 2915 |
| Wait-and-see cases | 64 | 100 | 100 | 0 | 2915 |
| Surgery for the primary tumor | 46 | 100 | 100 | 0 | 2614 |
Table 2: Summary of the post chemotherapy follow-up.
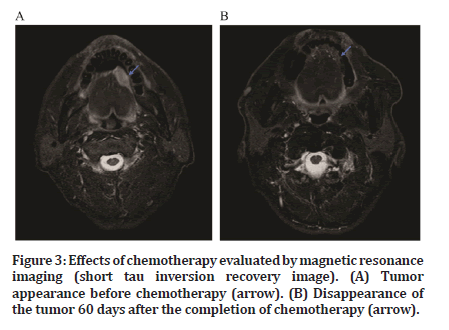
Figure 3:Effects of chemotherapy evaluated by magnetic resonance imaging (short tau inversion recovery image). (A) Tumor appearance before chemotherapy (arrow). (B) Disappearance of the tumor 60 days after the completion of chemotherapy (arrow).
Adverse events
The main adverse events were leukopenia, anemia, alopecia, oral mucositis, dysphagia, and nausea/ vomiting. Besides grade IV alopecia, all adverse events found were of grade I. No serious adverse events were observed (Table 3).
| Adverse events | Grade | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Leukopenia | 1 | 0 | 0 | 0 |
| Anemia | 1 | 0 | 0 | 0 |
| Alopecia | 0 | 0 | 0 | 8 |
| Dysphagia | 3 | 0 | 0 | 0 |
| Oral mucositis | 3 | 0 | 0 | 0 |
| Nausea/vomiting | 3 | 0 | 0 | 0 |
Table 3: Chemotherapy-associated adverse events graded according to the common terminology criteria for adverse events.
Post chemotherapy treatment
Two patients underwent surgery for the primary lesion after chemotherapy as initially planned, and the remaining six patients were followed up under the wait-and-see policy because they refused to undergo surgery (Table 1).
Prognosis
The follow-up period of the six patients who were managed under the wait-and-see policy ranged from 572 to 4334 days (median, 2915 days). Recurrence of the primary lesion occurred in one patient (Table 1). The 5-year DSS and RFS (organ-preserving) rates were both 83.3% (Figures 4 and 5). The follow-up periods of the two patients who underwent surgery after chemotherapy were 3745 and 1482 days (median, 2614 days), respectively, and primary lesion recurrence was not observed (Table 1). Furthermore, delayed cervical lymph node metastasis was not observed in any patient (Tables 1 and 2).
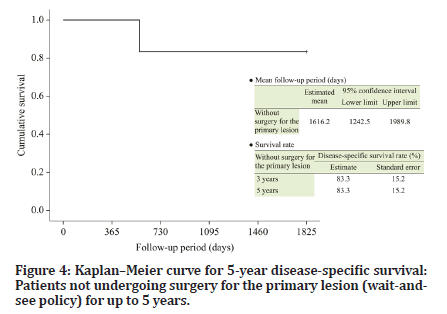
Figure 4:Kaplanâ??Meier curve for 5-year disease-specific survival: Patients not undergoing surgery for the primary lesion (wait-andsee policy) for up to 5 years.
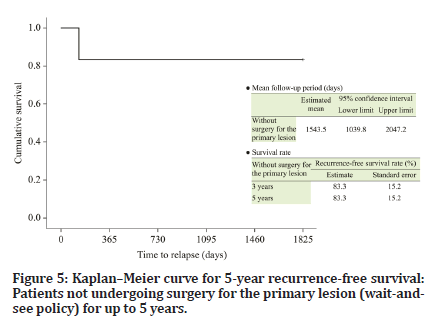
Figure 5:Kaplanâ??Meier curve for 5-year recurrence-free survival: Patients not undergoing surgery for the primary lesion (wait-andsee policy) for up to 5 years.
Discussion
The prognosis of patients with squamous cell carcinoma of the tongue differs according to the tumor DOI. The prognosis is poor even in stage I or II carcinomas of the tongue if DOI is greater than 4 mm, which also increases the risk of delayed metastasis to the cervical lymph nodes [2–4]. The National Comprehensive Cancer Network Guidelines also recommend cervical lymph node dissection for tumors with DOI >4 mm [5]. Therefore, comprehensive treatment is deemed necessary for patients with squamous cell carcinomas of the tongue with DOI >4 mm [4].
Considering the above information, we have introduced TPF chemotherapy for stage II squamous cell carcinoma of the tongue when DOI >4 mm as part of the comprehensive treatment. CDDP + 5-FU combined chemotherapy (i.e., PF chemotherapy) is considered the gold standard chemotherapy regimen for head and neck squamous cell carcinomas [10]; however, higher response rates have been reported with DTX + CDDP [11,12] and, more recently, with TPF chemotherapy [13]. Concomitant use of CDDP and DTX enhances the intratumoral accumulation of CDDP, thereby enhancing its effects [14]. The combination of DTX and CDDP has been reported to be particularly effective when administered via intra-arterial infusion, resulting in higher CR rates for the primary lesion [15].
Adverse events are a disadvantage of chemotherapy. However, in superselective intra-arterial chemotherapy, STS is administered via the superior vena cava so that CDDP can be neutralized before venous return, thereby minimizing the adverse events caused by CDDP and allowing the administration of higher doses of CDDP [16,17].
We attributed the absence of serious adverse events in this study to the benefits of CDDP neutralization achieved through superselective intra-arterial infusion. Superselective intra-arterial chemotherapy may be administered as chemotherapy with or without radiotherapy [17-19]. Almost all facilities treat superselective intra-arterial chemotherapy alone in resectable cases as preoperative adjuvant chemotherapy [16,19]. However, superselective intra-arterial chemo radiation therapy is frequently used as a radical treatment [17,18]. Our department has been administering TPF chemotherapy as induction chemotherapy, which has resulted in long-term RFS among patients who achieved CR and declined surgery. The 5-year DFS after surgery for primary stage I and II tongue carcinoma is 70%– 79.6% [20,21]. In this study, the 5-year RFS of patients who were managed under the wait-and-see policy was 83.3%, suggesting that TPF chemotherapy enabled organ preservation.
Occult cervical lymph node metastasis is reported to occur in 25%–50% of patients with stage II tongue carcinoma [22-25]. However, in this study, delayed cervical lymph node metastasis was not observed in any patient, suggesting that TPF chemotherapy effectively prevents cervical lymph node metastasis. Moreover, this effect was attributed to the superselective intra-arterial infusion of CDDP and DTX, which diverts treatment from the lymphatic flow to the sentinel lymph nodes to prevent occult cervical lymph node metastasis.
Over 90% of oral cancers are squamous cell carcinomas, and approximately 50%–60% of them are carcinomas of the tongue [1,21]. Controlling the primary lesion and cervical lymph node metastasis is key to improving the survival rate. Tongue cancers are mainly treated surgically. However, surgery for patients with stage II tongue cancer with DOI >4 mm can involve en bloc resection in glossectomy using the pull-through method and neck dissection, which can result in postoperative functional impairments. However, the outcomes of this study demonstrate the potential of TPF chemotherapy for use as a radical therapeutic option for stage II squamous cell carcinoma of the tongue with DOI >4 mm with the intent of preserving the tongue and preventing delayed cervical lymph node metastasis.
This study demonstrates the safety and efficacy of TPF chemotherapy in treating patients with stage II squamous cell carcinoma of the tongue with DOI >4 mm and providing good prognosis. However, this is a pilot study that included a small sample size; therefore, future studies involving a larger sample size are needed.
Acknowledgement
We express our sincere gratitude to all the neurosurgeons who have retired from the Department of Neurosurgery/ Head and Neck Surgery at Nihon University School of Dentistry at Matsudo. We have appreciated their cooperation in the treatment of oral cancer.
Conflict of Interest
The authors declare no conflicts of interest associated with this manuscript.
References
- Knežević A, Salamon T, Milankov M, et al. Assessment of quality of life in patients after lower limb amputation. Med Pregl 2015; 68:103-108.
- https://emedicine.medscape.com/article/1232102-overview
- Jadeja B, Zafer A, Jindal R, et al. Profile of lower limb amputees attended at a tertiary care hospital: A descriptive study. Int Multispecialty J Health 2016; 1:18-25.
- https://eric.ed.gov/?id=ED367122
- Legro MW, Reiber GD, Smith DG, et al. Prosthesis evaluation questionnaire for persons with lower limb amputations: Assessing prosthesis-related quality of life. Arch Phys Med Rehabil 1998; 79:931-938.
- Hussain MA, Al-Omran M, Salata K, et al. Population-based secular trends in lower-extremity amputation for diabetes and peripheral artery disease. CMAJ 2019; 191:E955-61.
- Alzahrani HA. Diabetes-related lower extremities amputations in Saudi Arabia: the magnitude of the problem. Annals Vasc Dis 2012; 1204160115.
- Badri MM, Tashkandi WA, Aldaqal SM, et al. Extremities amputations in King Abdulaziz University Hospital (2005-2009). JKAU Med Sci 2011; 18:13-25.
- Musa IR, Ahmed MO, Sabir EI, et al. Factors associated with amputation among patients with diabetic foot ulcers in a Saudi population. BMC Res Notes 2018; 11:1-5.
- Darter BJ, Hawley CE, Armstrong AJ, et al. Factors influencing functional outcomes and return-to-work after amputation: A review of the literature. J Occup Rehab 2018; 28:656-665.
- van der Schans CP, Geertzen JH, Schoppen T, et al. Phantom pain and health-related quality of life in lower limb amputees. J Pain Symptom Manage 2002; 24:429-436.
- Fisher K, Hanspal RS, Marks L. Return to work after lower limb amputation. Int J Rehabil Res 2003; 26:51-56.
- MacKenzie EJ, Bosse MJ, Kellam JF, et al. Early predictors of long-term work disability after major limb trauma. J Trauma Acute Care Surg 2006; 61:688-694.
- Hewson A, Dent S, Sawers A. Strength deficits in lower limb prosthesis users: A scoping review. Prosthet Orthot Int 2020; 44:323-340.
- Naidoo U, Ennion L. Barriers and facilitators to utilisation of rehabilitation services amongst persons with lower-limb amputations in a rural community in South Africa. Prosthet Orthot Int 2019; 43:95-103.
- Ennion L, Johannesson A. A qualitative study of the challenges of providing pre-prosthetic rehabilitation in rural South Africa. Prosthet Orthot Int 2018; 42:179-186.
- Davie-Smith F, Coulter E, Kennon B, et al. Factors influencing quality of life following lower limb amputation for peripheral arterial occlusive disease: A systematic review of the literature. Prosthet Orthot Int 2017; 41:537-547.
- Järnhammer A, Andersson B, Wagle PR, et al. Living as a person using a lower-limb prosthesis in Nepal. Disabil Rehabil 2018; 40:1426-1433.
- Whoqol Group. The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc Sci Med 1995; 41:1403-1409.
- Pezzin LE, Dillingham TR, MacKenzie EJ, et al. Use and satisfaction with prosthetic limb devices and related services. Arch Phys Med Rehabil 2004; 85:723-729.
- Srivastava K, Chaudhury S. Rehabilitation after amputation: Psychotherapeutic intervention module in Indian scenario. Scient World J 2014; 2014.
- Legro MW, Reiber GD, Smith DG, et al. Prosthesis evaluation questionnaire for persons with lower limb amputations: Assessing prosthesis-related quality of life. Arch Phys Med Rehab 1998; 79:931-938.
- Desmond DM, MacLachlan M. Factor structure of the trinity amputation and prosthesis experience scales (TAPES) with individuals with acquired upper limb amputations. Am J Phys Med Rehab 2005; 84:506-513.
- Pezzin LE, Dillingham TR, MacKenzie EJ. Rehabilitation and the long-term outcomes of persons with trauma-related amputations. Arch Phys Med Rehabil 2000; 81:292-300.
- Weisman IM, Zeballos RJ. Clinical exercise testing. Clin Chest Med 2001; 22:679-701.
- Datta D, Ariyaratnam R, Hilton S. Timed walking test—an all-embracing outcome measure for lower-limb amputees?. Clin Rehab 1996; 10:227-232.
- Smith DG. Amputations. In: Skinner BH (Ed.). Current diagnosis and treatment in orthopaedics. California: Lange, 2005.
- Akarsu S, Tekin L, Safaz I, et al. Quality of life and functionality after lower limb amputations: comparison between uni-vs. bilateral amputee patients. Prosthet Orthot Int 2013; 37:9-13.
- Eftekhari N. Amputation rehabilitation: In: O’Young B, Young MA, Stiens SA (Eds). PMR secrets. Philadelphia: Hanley & Belfus, 1999; 214–222.
- Demirsoy C. The MOS-SF-36 health survey: A validation study with a Turkish sample. Master’s Thesis, University of Bosphorus, Turkey, 1999.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Shigeo Tanaka1*, Maya Oshima1, Hideo Niwa2, Yasuhide Makiyama2, Kayo kuyama3, Hirayama4 and Masamichi Komiya1
1Department of Oral Surgery, Nihon University School of Dentistry at Matsudo, Matsudo Chiba 271-8587, Japan2Department of Neurosurgery/Head and Neck Surgery, Nihon University School of Dentistry at Matsudo, Matsudo Chiba 271-8587, Japan
3Department of Pathology, Nihon University School of Dentistry at Matsudo, Matsudo Chiba 271-8587, Japan
4Department of Orofacial and Head Pain Clinic, Nihon University Hospital at Matsudo, Chiba 271-8587, Japan
Citation: Shigeo Tanaka, Maya Oshima, Hideo Niwa, Yasuhide Makiyama, Kayo Kuyama, Teruyasu Hirayama, Masamichi Komiya, Pilot Study on the Therapeutic Effects of Cisplatin and Docetaxel Superselective Intra-Arterial Infusion and Systemic 5-Fluorouracil Combination Chemotherapy for Stage II Squamous Cell Carcinoma of the Tongue with Greater than 4 mm Depth of Invasion, J Res Med Dent Sci, 2022, 10 (7):13-17.
Received: 26-Jun-2022, Manuscript No. JRMDS-22-67743; , Pre QC No. JRMDS-22-67743 (PQ); Editor assigned: 28-Jun-2022, Pre QC No. JRMDS-22-67743 (PQ); Reviewed: 13-Jul-2022, QC No. JRMDS-22-67743; Revised: 17-Jul-2022, Manuscript No. JRMDS-22-67743 (R); Published: 24-Jul-2022
