Research - (2022) Volume 10, Issue 3
Potential compounds from Ceiba pentandra pods against Candidiasis by Candida albicans and Candida tropicalis
*Correspondence: Mohammed Sarosh Khan, Department of Basic Medical Sciences, College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj-11942, Saudi Arabia, Email:
Abstract
Invasive candidiasis is a dangerous infection that can harm the blood, the heart, the brain, the eyes, the bones, and other bodily organs. Candidemia, a Candida-related bloodstream infection, is a prevalent illness among hospitalized patients. It might be difficult to tell whether symptoms are attributable to a Candida infection in people who have invasive candidiasis since they are often unwell from other medical issues. Candida albicans and Candida tropicalis are the most prevalent Candida species that cause infection in humans. Occurrence of antimicrobial resistance in candidal pathogens led to difficult in treatment of such infections. Consequently, there remains a strong need for new antimicrobial agents. Therefore, the present study focuses on evaluating the anti-candidal activity of the Ceiba pentandra pod extracts against candidiasis causing C. albicans and C. tropicalis. Bioactive compounds of the pods were extracted using methanol as solvent. DPPH radical scavenging activity and ferric reducing antioxidant power assay were performed. The pod extracts showed evident anti-candidal activity and lower MIC levels of 31.5 mg and 15.6 mg against C. albicans and C. tropicalis. Further, pod extracts also showed better antioxidant values, 68.31 ± 0.43% of DPPH scavenging and 61.8 ± 0.87% of reducing power was observed on 1000 µg of extract. From the analysis, the bioactive compounds from Ceiba pentandra pods showed significant candidal inhibition activity and therefore can be used for treatment of candidiasis.
Keywords
Candidiasis, Ceiba pentandra pod, Anti-candidal activity, Minimum inhibitory concentration, Anti-oxidant activity
Introduction
Candidiasis is a fungal infection caused by Candida species. Candida albicans and Candida tropicalis are the most prevalent Candida species that cause infection in humans [1]. Candida may survive on the skin and within the body, in locations including the mouth, throat, stomach, and vaginal canal, without creating difficulties. If Candida gets out of hand or gets deep into the body, it can cause illnesses (for example, the bloodstream or internal organs like the kidney, heart, or brain). Fever, chills, a weakened immune system, and gastrointestinal issues are all possible side effects [2].
Candidiasis is characterised by the site of infection. The term "vaginal yeast infection" refers to candidiasis in the vaginal area. "Vaginal candidiasis," "vulvovaginal candidiasis," and "candidal vaginitis" are some of the other names for this condition. If the environment within the vagina alters in a way that favours Candida growth, it can multiply and produce an infection [3]. Vaginal itching or soreness, pain or discomfort when peeing, and atypical vaginal discharge are all indications of vaginal candidiasis. Although most cases of vaginal candidiasis are minor, some women may experience severe infections with redness, swelling, and fissures in the vaginal wall [4].
Invasive candidiasis is a dangerous infection that can harm the blood, the heart, the brain, the eyes, the bones, and other bodily organs. Candidemia, a Candida-related bloodstream infection, is a prevalent illness among hospitalised patients [5]. It might be difficult to tell whether symptoms are attributable to a Candida infection in people who have invasive candidiasis since they are often unwell from other medical issues. Fever and chills that do not improve following antibiotic therapy for suspected bacterial infections are the most prevalent signs of invasive candidiasis. If the infection spreads to other regions of the body, such as the heart, brain, eyes, bones, or joints, further symptoms may appear [6].
Thrush, or oropharyngeal candidiasis, is a kind of candidiasis that affects the mouth and throat. Oesophageal candidiasis, also known as Candida esophagitis, is a kind of candidiasis that affects the oesophagus (the tube that links the throat to the stomach). One of the most prevalent illnesses among HIV/AIDS patients is esophageal candidiasis [7]. White spots on the inner cheeks, tongue, roof of mouth, and throat are some of the signs of candidiasis in the mouth and throat (photo showing candidiasis in the mouth), Redness or soreness in the mouth, a cotton-like sensation in the mouth, a loss of taste, pain while eating or swallowing, and cracking and redness at the corners of the mouth are all symptoms to look out for. The most common symptoms of candidiasis in the oesophagus are discomfort and trouble swallowing [8].
The authors report that 1,400 instances of ICU acquired candidemia in India, with 65 % of the patients being adults. The prevalence varied by ICU in different nations, ranging from 0.24 to 34.3 individuals per 1,000 ICU admissions. Candidaemia patients' 30-day crude and attributable death rates were 44.7 and 19.7%, respectively [9]. A frequency of 6.51 cases per 1,000 ICU admissions was found in the first investigation of Candida bloodstream infection in 27 Intensive Care Units (ICU) in India, corresponding to 90,000 cases nationwide. About 40,000 people died, with mortality rates ranging from 35 to 75%. In India, over 14.3 million patients are hospitalised to intensive care units each year [10].
The antibiotic resistance is concerned as a threat to modern medicine. Prevention has been on reducing unnecessary antibiotic prescription in order to reduce resistance [11]. Despite some positive developments, existing resistance is proving difficult to overcome; moreover, new mechanisms of resistance evolve to form and proliferate worldwide. Consequently, there remains a strong need for new antibiotics [12].
Ceiba pentandra is a tropical rainforest emergent tree that is typically regarded as magnificent. It's known as Kapok in the area, and it's the cotton-like fluff that comes from the seedpods. Ceiba pentandra is also known as the Kapok tree, Ceiba de Lana, silk-cotton tree, and other names. Ceiba pentandra is a tropical tree native to Mexico, Central America and the Caribbean, northern South America, and West Africa (as the variation C. pentandra var guineensis) [13]. It belongs to the Malvales order and the Malvaceae family (formerly in the Bombacaceae family). It is planted in South and Southeast Asia, where a smaller variant was introduced. In English, the tree and the cotton-like fluff collected from its seed pods are known as kapok, a Malay-derived word that originally applied to Bombax ceiba, a tropical Asian native. The tree is known as "ceiba" in Spanish-speaking nations [14]. As there were few reports on antimicrobial activity of the Ceiba pentandra leaf extracts, no studies were reported on anti-candidal activity of Ceiba pentandra pod extracts. Therefore, the present study focuses on evaluating the anti-candidal activity of the Ceiba pentandra pod extracts against candidiasis causing C. albicans and C. tropicalis. DPPH radical scavenging activity and ferric reducing antioxidant power assay of the extracts were performed.
Materials and Methods
Collection of Ceiba pentandra
The dry pods of Ceiba pentandra were collected and stored at room temperatures (Figure 1). The pods were dried in shade under room temperature, purely grounded to powders and kept in sterile container for extraction.

Figure 1: Ceiba pentandra pods used in the study.
Extraction of bioactive compounds using soxhlet apparatus
Powders of Ceiba pentandra pods were positioned in a porous bag or “thimble” made from a cellulose or strong filter paper, which is placed in thimble chamber of the Soxhlet apparatus. The extractor had been filled with solvent solution of methanol and the temperature of 60°C was set and left for 6 hours. The extracts were procured and the solvents were vaporized. The dried extracts were procured and kept in sterile containers.
Anti-oxidant activity
DPPH radical scavenging assay
The antioxidant activity of the extracted plant extract from Ceiba pentandra pods was assessed based on ability of donate hydrogen or scavenging radicals, by with the stable radical 2,2-diphenyl- 1-picrylhydrazyl (DPPH), according to procedure described by Blois (1958) [15]. The extracts at different concentrations (100 – 1000 μg) were taken and the total volume was adjusted to 100 μl with methanol. About 5 ml of 0.1 mM methanol solution of DPPH was mixed and permitted to stand for 20 min at 27°C. The absorbance (OD values) of the extract was measured at 517 nm. Percentage radical scavenging activity of the sample was calculated as follows: The assay was performed in triplicates and the results were determined in mean ± SD.
Ferric reducing antioxidant power (FRAP) assay
The Ferric reducing antioxidant power (FRAP) assay was used to assess the reducing ability of the extract, according to the technique described by Benzie and Strain, 1996 [16]. The FRAP reagent containing 2.5 ml of a 10 mM TPTZ solution in 40 mM HCl, 2.5 ml of 20 mM FeCl3.6H2O and 25 ml of 300 mM acetate buffer (pH 3.6) was prepared and kept at 37°C. 900 μl FRAP reagent was mixed with 90 μl water and 10 μl of the sample. The reaction mixture was set to stand for 30 minutes at 37°C and the absorbance (OD values) was measured at 593 nm.
Anti-candidal activity using well diffusion method
The anti-candidal effectiveness of the plant extract was estimated against the clinical pathogens by well diffusion method. Muller Hinton Agar was prepared and sterilized, and poured into plates. Overnight cultures of test pathogens were cultured and 0.1% of culture solution of every test organisms were streaked all over the petri plate with the sterile cotton swab by rotating the plate at 60º angle for every streaking. 6 mm well borer was used to bore wells on the agar surface of every NA plates. About 100 μl of the samples were filled into the well and the plates were incubated in an incubator at 370C for 48 h. The anti-candidal activity was determined in terms of inhibitory zones around the wells loaded with samples in all the MH Agar plates containing test pathogens. The obtained clear zones were detected and calculated in millimetre (mm).
Evaluating minimum inhibitory concentration (MIC) of the plant extracts
Slight modification in the dilution method for the determination of MIC was carried out in this study. Plant extracts was diluted into various concentrations (1.95 mg/ml, 3.9 mg/ml, 7.8 mg/ ml, 15.6 mg/ml, 31.5 mg/ml, 62.5 mg/ml, 125 mg/ml and 250 mg/ml, in 1 ml of sterile nutrient broth in test tubes. A 100 μl of E. coli culture at 0.5 McFarland standards (Eucast, 2003) [17] was inoculated to the tubes. Correspondingly, this was repeated for S. aureus. The tubes were incubated at 37°C for 24 h and observed for growth or turbidity by using an unaided eye (CLSI, 2012) [18].
Results
Antioxidant assay
DPPH radical scavenging assay
DPPH radical scavenging activity of Ceiba pentandra pod extracts was evaluated by using ascorbic acid as standard. Five concentrations of the pod extracts were used (100 μg, 250 μg, 500 μg, 750 μg and 1000 μg). 100 μg of plant extracts showed 8.08 ± 1.19% of DPPH scavenging, 14.7 ± 0.51% for 250 μg, 30.88 ± 0.72% for 500 μg, 50.73 ± 1.26% for 750 μg and 68.31 ± 0.43% for 1000 μg. Similarly, ascorbic acid at showed 43.21 ± 0.22%, 64.52 ± 0.49%, 72.23 ± 1.08%, 83.77 ± 0.15% and 91.75 ± 0.78% of DPPH scavenging for 100 μg, 250 μg, 500 μg, 750 μg and 1000 μg. Figure 2 shows the graphical representation of the DPPH radical scavenging activity of Ceiba pentandra pod extract.
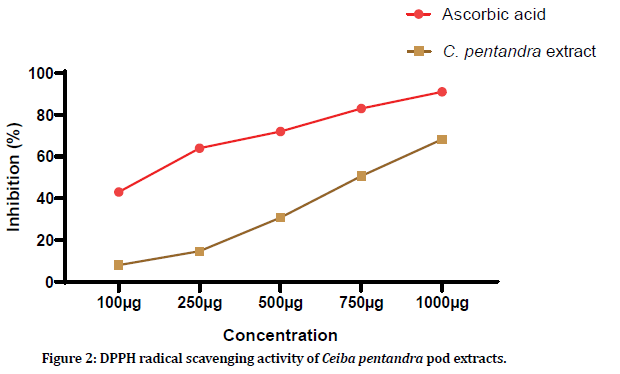
Figure 2:DPPH radical scavenging activity of Ceiba pentandra pod extracts.
FRAP reducing power
The Ferric reducing anti-oxidant power of the pod extract was evaluated using 5 different concentrations. 100 μg, 250 μg, 500 μg, 750 μg and 1000 μg of plant extracts showed 19.34 ± 0.72%, 33.66 ± 0.44%, 41.7 ± 1.66%, 59.29 ± 0.52%, and 61.8 ± 0.87% of reducing power. Whereas standard ascorbic acid showed 48.87 ± 0.66%, 63.69 ± 1.12%, 68.35 ± 0.45%, 85.02 ± 0.1.36% and 95.32 ± 1.04% of reducing power. Figure 3 shows the graphical representation of reducing power of the Ceiba pentandra pod extract.
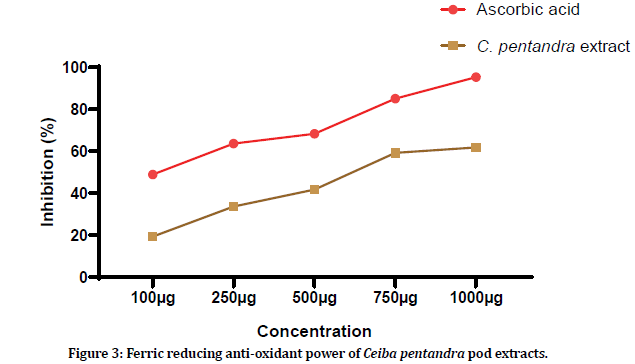
Figure 3:Ferric reducing anti-oxidant power of Ceiba pentandra pod extracts.
Anti-candidal analysis
Anti-candidal activity of the Ceiba pentandra pod extract was evaluated against Candida albicans and Candida tropicalis. Four concentrations (125 mg/ml, 250 mg/ml, 500 mg/ml and 1000 mg/ ml) of the pod extracts were used (Figures 4 and Figure 5). Pod extracts at 125 mg/ml, 250 mg/ ml, 500 mg/ml and 1000 mg/ml showed 8.66 ± 0.57 mm, 12.33 ± 0.57 mm, 13.33 ± 1.15 mm and 17.33 ± 0.57 against C. albicans and 10.00 ± 1.00 mm, 13.33 ± 0.57 mm, 15.66 ± 0.57 mm and 20.33 ± 1.15 mm against C. tropicalis (Figure 6).
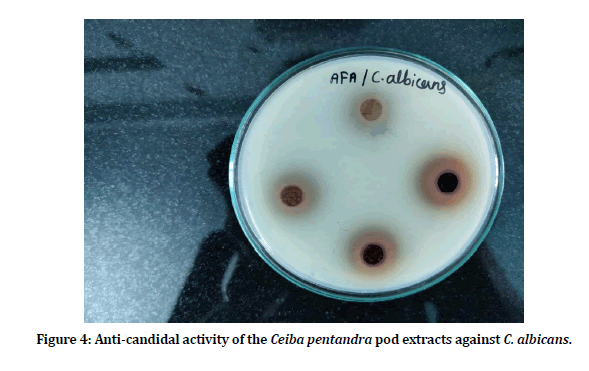
Figure 4: Anti-candidal activity of the Ceiba pentandra pod extracts against C. albicans.
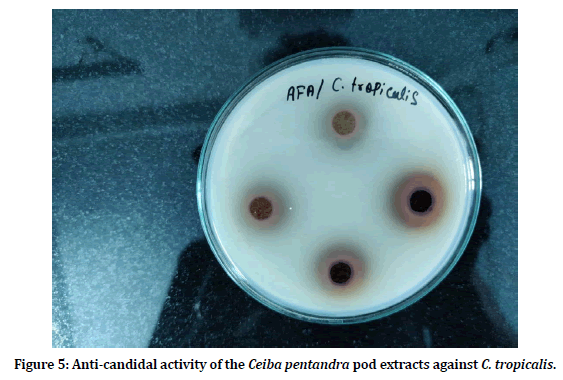
Figure 5: Anti-candidal activity of the Ceiba pentandra pod extracts against C. tropicalis.<
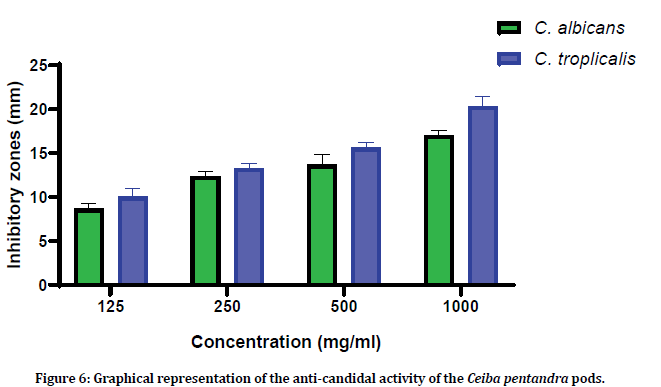
Figure 6: Graphical representation of the anti-candidal activity of the Ceiba pentandra pods.
Minimum inhibitory concentration
Minimum inhibitory concentration is the defined as the least concentration required inhibiting the growth of the test pathogens. MIC of the Ceiba pentandra pod extracts was evaluated by using broth dilution method. The tubes were observed for candida growth and the concentration in which no growth seen is estimated as the MIC. Figures 7 and Figure 8 shows the MIC evaluation of pod extracts against candidal pathogens.
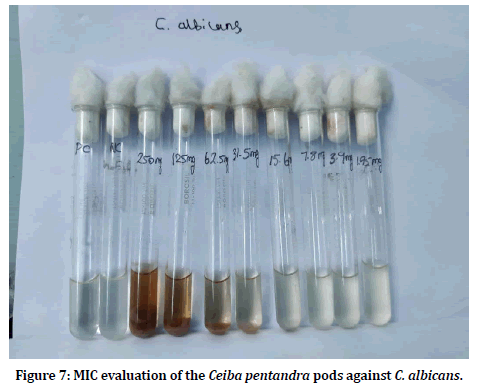
Figure 7:MIC evaluation of the Ceiba pentandra pods against C. albicans.
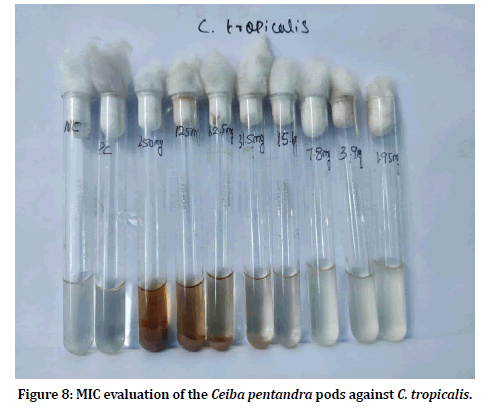
Figure 8:MIC evaluation of the Ceiba pentandra pods against C. tropicalis.
Discussion
Ceiba pentandra is a massive tree with a trunk diameter of up to 3 metres (9.8 feet) and a height of up to 70 metres (230 ft.). The main stem and branches are densely covered in thorns. The leaflets of kapok leave range from 5 to 9, and the leaf length is roughly 20 cm (7.9 in). Hundreds of pods with a length of 15 cm (5.9 in) and a diameter of 2–5 cm (1–2 in) are produced by the Kapok tree [19]. Flowers are in clusters at the ends of branchlets, white or whitish-yellow. Flowering is in January-April. Fruit is an ellipsoid to fusiform capsule, indehiscent, valves with silky fibres; seeds numerous, subglobose, enveloped in silky cotton. The seeds are contained in the pods, which are puffy in the surrounding regions. A yellowish fibre made up of cellulose and lignin can also be found in the pod. Ceiba pentandra is distinguished from other species by the pattern and size of its conical spines [20].
Ceiba pentandra is an elegant large tree, found throughout hotter parts of India, especially in southern and western India and the Andaman Islands and Indonesia, Malaysia, South and Central America, Mexico, West Africa, and Caribbean islands, among other places. It has a lengthy tradition of usage in the treatment of liver and spleen illnesses, as well as digestive fire, constipation, and blood diseases. Gum is used to cure bed wetting in children and diarrhea because of its typic character [21]. The leaves are both unctuous and stypic in appearance. This plant's bark and leaves are used to treat diabetes, dizziness, headaches, hypertension, and fever.
Flowers decoction works as a laxative and can be used to treat constipation. Its roots are utilized in the treatment of edema and ascites because to its diuretic properties.
In the present study pods extracts of the Ceiba pentandra was used to evaluate the anticandidal activity against Candidiasis causing Candida albicans and Candida tropicalis. Thrush and other types of candidiasis are caused mostly by Candida albicans, an opportunistic fungal infection. Diploid Candida albicans has no sexual cycle and alternates between yeast, mycelial, and pseudomycelial forms [22-24]. The relevance of these distinct morphologies in pathogenesis have been debated, however hyphal differentiation has recently been related to systemic virulence and C. albicans cells' capacity to evade macrophages. Filamentous forms are also better at infiltrating epithelial cells and agar surfaces in vitro than yeast forms. This might be due to hyphal forms' mechanical advantages in penetrating solid substrates, as well as the synthesis of hyphaspecific hydrolytic enzymes like those of the secreted aspartyl proteinases, which appear to contribute to virulence [8].
Candida tropicalis is a yeast type of fungus that is pathogenic in neutropenic hosts and spreads to peripheral organs through the circulation. C. tropicalis is a diploid dimorphic yeast that lives as either ellipsoidal budding cells or a pseudomycelium consisting of long, branching components bearing conidia alone, in short chains, or clusters. Invasive candidiasis is more common in some areas than others, generating 3–66% of candidemia. C. tropicalis is closely related to Candida albicans in terms of taxonomy, and they share many pathogenic characteristics. It is especially dangerous in neutropenic hosts, where haematogenous seeding of peripheral organs is widespread [25].
In candidiasis, antifungal drug resistance (ADR) and fungal virulence are major concerns for the host-parasite interaction. Although azoles, notably fluconazole (FLC), are still among the most widely used antifungal medicines, their widespread use in clinical practise for both therapy and prevention has promoted the establishment of resistance strains. The prospect of azole drug resistance, along with the relative shortage of antifungal treatments, encouraged the emergence of new medications [26]. The pod extracts showed evident anti-candidal activity and lower MIC levels of 31.5 mg and 15.6 mg against C. albicans and C. tropicalis. Further, pod extracts also showed significant antioxidant values, 68.31 ± 0.43% of DPPH scavenging and 61.8 ± 0.87% of reducing power was observed on 1000 μg of extract.
The Ceiba pentandra plant's crude extracts have potential antimicrobial properties. Antimicrobial studies of the extracts at relatively high concentrations revealed effectiveness against test bacteria, according to Anosike et al. 2012 [27]. The methanol extract of leaves was active against all of the test isolates, with the fungus Candida albicans and Aspergillus niger showing the most activity. It was also more active against Gram positive bacteria than against Gram negative bacteria. On Escherichia coli and Bacillus subtilis, the ethanol extract of leaves displayed increased activity, whereas on fungus, it had negligible action. Only E. coli and B. subtilis demonstrated activity in the petroleum ether extract of the leaves.
Ravi Kiran et al., 2015 [28] observed during seed germination, the phenolic content increased corresponding to the increased antioxidant activity. The antioxidant capacity of phenolic compounds is determined by their structure, in particular the ease with which a hydrogen atom from an aromatic hydroxyl group can be donated to the free radicals. These above findings well correlate with the present study.
Phytochemical study of the plant extracts revealed flavonoids, tannins, steroids, alkaloids, and glycosides, among other bioactive components. The quantities of phytochemicals in the various extracts varied, with methanol and ethanol having almost the same quantity of phytochemicals [29]. Only large concentrations of lipid derivatives were found in the petroleum ether extract. Phenolics, or phenolic acids, are crucial intermediates in phenylpropanoid metabolism in plant cells, tissues, and organs. They are also known to have a role in growth control, as well as the differentiation and organogenesis processes. Biological properties include, bioactive compounds are capable of modulating metabolic processes and demonstrate positive properties such as antioxidant effect, inhibition of receptor activities, inhibition or induction of enzymes, and induction and inhibition of gene expression. Bioactive compounds (BACs) with antimicrobial activity are utilized for years as antimicrobial agents (AMAs) in the form of pure substances and plant extracts [29].
Conclusion
Anti-candidal activity of the Ceiba pentandra pod extracts against C. albicans and C. tropicalis was determined in this present study. Bioactive compounds from Ceiba pentandra pods were extracted using methanol as solvent. Anticandidal activity was investigated by well diffusion and broth dilution method. From the analysis, pod extracts showed evident candidal inhibition activity. Along with inhibition, other biological properties i.e. anti-oxidant activity was also evaluated. Pod extract showed significant DPPH radical scavenging and reducing power. In addition, further studies were required to purify the bioactive compounds and identify the compounds exerting anti-candidal actions for development of effective antibiotics irrespective of AMR.
Conflict of Interest
The author declares no conflict of interest.
Acknowledgement
The author is grateful to the Deanship of Scientific Research, Prince Sattam bin Abdulaziz University, Al-Kharj, Saudi Arabia for its support for this research work.
References
- Bhattacharya S, Sae-Tia S, Fries BC. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020; 9:312.
- Peters BM, Coleman BM, Willems HM, et al. The interleukin (IL) 17R/IL-22R signaling axis is dispensable for vulvovaginal candidiasis regardless of estrogen status. J Infectious Dis 2020; 221:1554-1563.
- Rosati D, Bruno M, Jaeger M, et al. Recurrent vulvovaginal candidiasis: An immunological perspective. Microorganisms 2020; 8:144.
- Azie N, Angulo D, Dehn B, et al. Oral Ibrexafungerp: An investigational agent for the treatment of vulvovaginal candidiasis. Expert Opin Investig Drugs 2020; 29:893-900.
- Walsh TJ, Katragkou A, Chen T, et al. Invasive candidiasis in infants and children: Recent advances in epidemiology, diagnosis, and treatment. J Fungi 2019; 5:11.
- Pappas PG, Lionakis MS, Arendrup MC, et al. Invasive candidiasis. Nature Rev Dis Primers 2018; 4:1-20.
- Olum R, Baluku JB, Okidi R, et al. Prevalence of HIV-associated esophageal candidiasis in sub-Saharan Africa: A systematic review and meta-analysis. Tropical Med Health 2020; 48:1-0.
- Hellstein JW, Marek CL. Candidiasis: Red and white manifestations in the oral cavity. Head Neck Pathol 2019; 13:25-32.
- Ann Chai LY, Denning DW, Warn P. Candida tropicalis in human disease. Crit Rev Microbiol 2010; 36:282-98.
- Angiolella L, Stringaro AR, De Bernardis F, et al. Increase of virulence and its phenotypic traits in drug-resistant strains of Candida albicans. Antimicrob Agents Chemother 2008; 52:927-36.
- Shivarathri R, Jenull S, Stoiber A, et al. The two-component response regulator Ssk1 and the mitogen-activated protein kinase Hog1 control antifungal drug resistance and cell wall architecture of Candida auris. M Sphere 2020; 5:e00973-20.
- Wu Y, Grossman N, Totten M, et al. Antifungal susceptibility profiles and drug resistance mechanisms of clinical Lomentospora prolificans isolates. Antimicrob Agents Chemother 2020; 64:e00318-20.
- Wédjangnon AA, Sourou Kuiga NB, Houêtchégnon T, et al. Spatial distribution and interspecific association patterns between Mansonia altissima A. Chev., Ceiba pentandra (L.) Gaertn and Triplochiton scleroxylon K. Schum. in a moist semi-deciduous forest. Ann For Sci 2020; 77:1-1.
- Kusumaningtyas RD, Utomo MY, Nurjanah PR, et al. Synthesis of biodiesel from kapok (Ceiba pentandra L.) seed oil through ultrasound-enhanced transesterification reaction. Int J Chemtech Res 2016; 9:627–634.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181:1199–1200.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 1996; 239:70-76.
- Debast SB, Bauer MP, Kuijper EJ. European society of clinical microbiology and infectious diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin Microbiol Infect 2014; 20:1-26.
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. Fourth Inf. Wayne, Pennsylvania 2012.
- Jamaluddin NA, Riayatsyah TM, Silitonga AS, et al. Techno-economic analysis and physicochemical properties of Ceiba pentandra as second-generation biodiesel based on ASTM D6751 and EN 14214. Processes 2019; 7:636.
- Adeniji IT, Olomola DB, Jegede OC. Seed germination and seedling growth of Ceiba Pentandra (L) as influenced by different soil types in Ibadan, Southwest Nigeria. J Res Fores Wildlife Environ 2019; 11:316-20.
- Ong HC, Milano J, Silitonga AS, et al. Biodiesel production from Calophyllum inophyllum-Ceiba pentandra oil mixture: Optimization and characterization. J Clean Prod 2019; 219:183-98.
- Appiah KS, Mardani HK, Osivand A, et al. Exploring alternative use of medicinal plants for sustainable weed management. Sustainability 2017; 9:1468.
- Baumgardner DJ. Oral fungal microbiota: To thrush and beyond. J Patient Cent Res Rev 2019; 6:252-261.
- Pao-Yu Chen, Yu-Chung Chuang, Un-In Wu, et al. Clonality of fluconazole-no susceptible Candida tropicalis in bloodstream infections, Taiwan, 2011–2017. Emerg Infect Dis 2019; 25:1668.
- Lee Y, Puumala E, Robbins N, et al. Antifungal drug resistance: Molecular mechanisms in Candida albicans and beyond. Chem Rev 2020; 121:3390-411.
- Anosike CA, Ogili OB, Nwankwo ON, et al. Phytochemical screening and antimicrobial activity of the petroleum ether, methanol and ethanol extracts of Ceiba pentandra stem bark. J Med Plants Res 2012; 6:5743-7.
- Kiran CR, Rao DB, Sirisha N, et al. Assessment of phytochemicals and antioxidant activities of raw and germinating Ceiba pentandra (kapok) seeds. J Biomed Res 2015; 29:414.
- Pramono A, Bustamam N, Amalia M, et al. Immense addition of royal jelly apis mellifera (ceiba pentandra) insufficient to increase fibroblast preputium proliferation. In IOP Conference Series: Materials Science and Engineering 2019.
- Saliu TP, Umar HI, Ogunsile OJ, et al. Molecular docking and pharmacokinetic studies of phytocompounds from Nigerian medicinal plants as promising inhibitory agents against SARS-CoV-2 methyltransferase (nsp16). J Genetic Eng Biotechnol 2021; 19:1-2.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Author Info
Department of Basic Medical Sciences, College of Medicine, Prince Sattam Bin Abdulaziz University, Al-Kharj-11942, Saudi ArabiaCitation: Mohammed Sarosh Khan, Potential compounds from Ceiba pentandra pods against Candidiasis by Candida albicans and Candida tropicalis, J Res Med Dent Sci, 2022, 10 (3):70-78.
Received: 07-Feb-2022, Manuscript No. JRMDS-22-53667; , Pre QC No. JRMDS-22-53667 (PQ); Editor assigned: 09-Feb-2022, Pre QC No. JRMDS-22-53667 (PQ); Reviewed: 16-Feb-2022, QC No. JRMDS-22-53667; Revised: 22-Feb-2022, Manuscript No. JRMDS-22-53667 (R); Published: 01-Mar-2022
